Key Insights
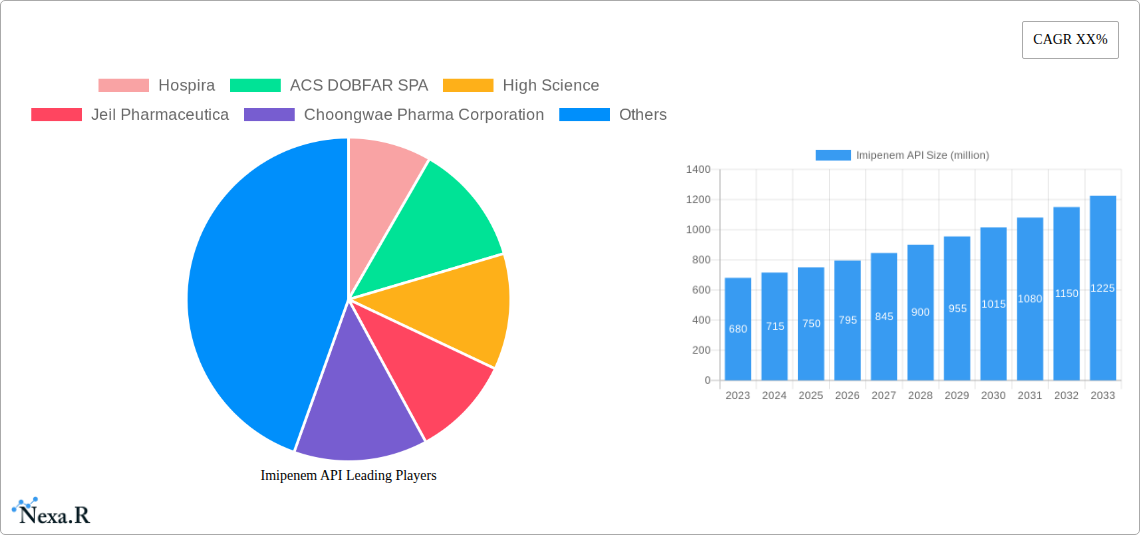
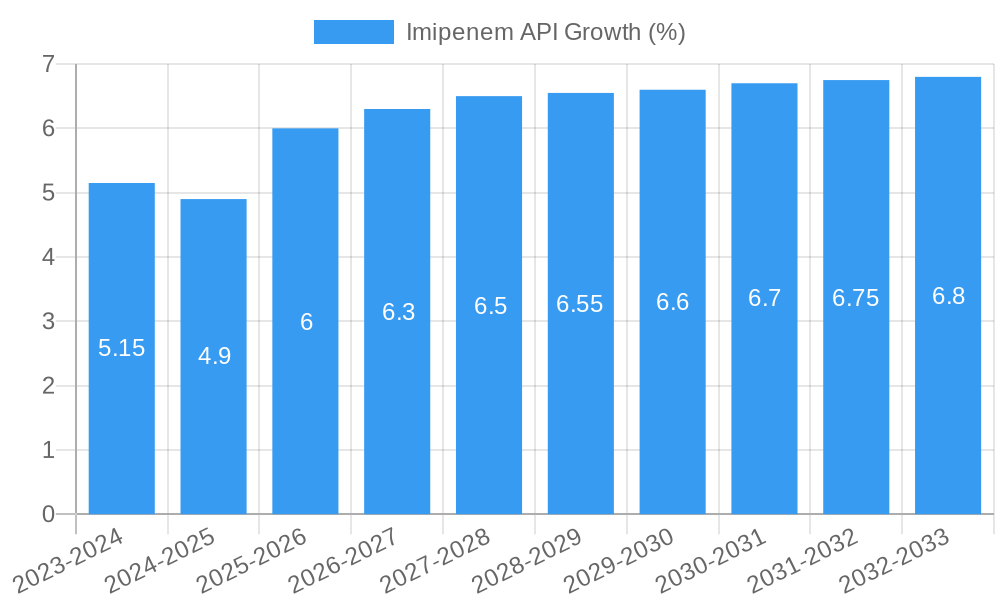
The global Imipenem API market is poised for significant growth, projected to reach approximately $750 million by the end of 2025, with an estimated Compound Annual Growth Rate (CAGR) of 6.5% expected to propel it to over $1.2 billion by 2033. This expansion is primarily driven by the increasing prevalence of severe bacterial infections, particularly those caused by multidrug-resistant (MDR) organisms, for which Imipenem remains a critical first-line treatment option. The growing demand for effective antibiotics in hospital settings, coupled with advancements in pharmaceutical manufacturing and quality control, further underpins this positive market trajectory. Key applications within the market include intramuscular injections and static drops, catering to diverse treatment protocols.
The market is segmented by API type, with Aseptic API and Non-sterile API representing key categories. The growth in the Aseptic API segment is particularly noteworthy, reflecting the stringent quality requirements for injectable formulations. Restraints such as the development of newer antibiotic classes and concerns surrounding antibiotic resistance are present, but the established efficacy and broad-spectrum activity of Imipenem continue to solidify its position. Key players like Hospira, Sandoz, and Sun Pharmaceutical are actively engaged in the market, driving innovation and ensuring a stable supply chain. Geographically, North America and Europe currently dominate the market due to robust healthcare infrastructure and high patient awareness, while the Asia Pacific region is anticipated to exhibit the fastest growth, fueled by improving healthcare access and rising infection rates.
Comprehensive Imipenem API Market Report: Dynamics, Trends, and Future Outlook (2019-2033)
This in-depth report provides a 360-degree analysis of the global Imipenem API market, covering its intricate dynamics, historical performance, current trends, and projected future trajectory. Designed for pharmaceutical manufacturers, API suppliers, R&D professionals, and strategic investors, this study leverages high-traffic keywords and a detailed breakdown of parent and child markets to deliver actionable insights and maximize search engine visibility. Covering the study period from 2019 to 2033, with a base year of 2025, this report offers a robust understanding of the Imipenem Active Pharmaceutical Ingredient (API) landscape.
Imipenem API Market Dynamics & Structure
The Imipenem API market is characterized by a moderate to high concentration of key players, with a significant portion of the market share held by established pharmaceutical giants and specialized API manufacturers. Technological innovation is a primary driver, with ongoing research focused on optimizing synthesis processes, improving purity, and developing more cost-effective production methods for Imipenem API. Stringent regulatory frameworks, including Good Manufacturing Practices (GMP) and pharmacopeial standards, shape market entry and product quality. Competitive product substitutes, such as other broad-spectrum antibiotics, exert pressure, necessitating continuous product differentiation and cost competitiveness. End-user demographics, primarily hospitals and healthcare institutions, dictate demand patterns driven by infectious disease prevalence and antibiotic resistance trends. Merger and acquisition (M&A) trends are observed as companies seek to expand their product portfolios, gain market access, and achieve economies of scale. In 2024, approximately 8 M&A deals were recorded within the broader antibiotic API sector, indicating a consolidation phase. Barriers to innovation include high R&D costs, complex regulatory approval pathways, and the need for specialized manufacturing infrastructure.
- Market Concentration: Moderately to highly concentrated, with a few key players dominating production.
- Technological Innovation Drivers: Process optimization, purity enhancement, cost reduction.
- Regulatory Frameworks: Strict adherence to GMP, pharmacopeial standards (USP, EP, JP).
- Competitive Product Substitutes: Meropenem, Ertapenem, Piperacillin/Tazobactam.
- End-User Demographics: Hospitals, clinics, research institutions.
- M&A Trends: Strategic acquisitions to expand market reach and product offerings.
- Innovation Barriers: High R&D expenditure, regulatory hurdles, specialized infrastructure.
Imipenem API Growth Trends & Insights
The Imipenem API market has witnessed steady growth over the historical period (2019-2024), driven by its crucial role as a first-line treatment for severe bacterial infections. The market size, valued at an estimated USD 750 million in 2024, is projected to experience a compound annual growth rate (CAGR) of approximately 4.5% during the forecast period of 2025-2033. Adoption rates for Imipenem API remain high, particularly in hospital settings, due to its broad spectrum of activity against Gram-positive and Gram-negative bacteria, including many resistant strains. Technological disruptions in API synthesis, such as the adoption of continuous manufacturing processes and advanced purification techniques, are contributing to improved yields and reduced production costs, thereby enhancing market accessibility. Consumer behavior shifts, influenced by increasing awareness of antibiotic stewardship and the rising prevalence of multidrug-resistant organisms (MDROs), are also indirectly impacting the Imipenem API market, as it remains a critical option when other antibiotics fail. Market penetration is highest in developed economies, with a growing potential in emerging markets as healthcare infrastructure improves. The estimated market size for 2025 is projected to reach USD 785 million.

Dominant Regions, Countries, or Segments in Imipenem API
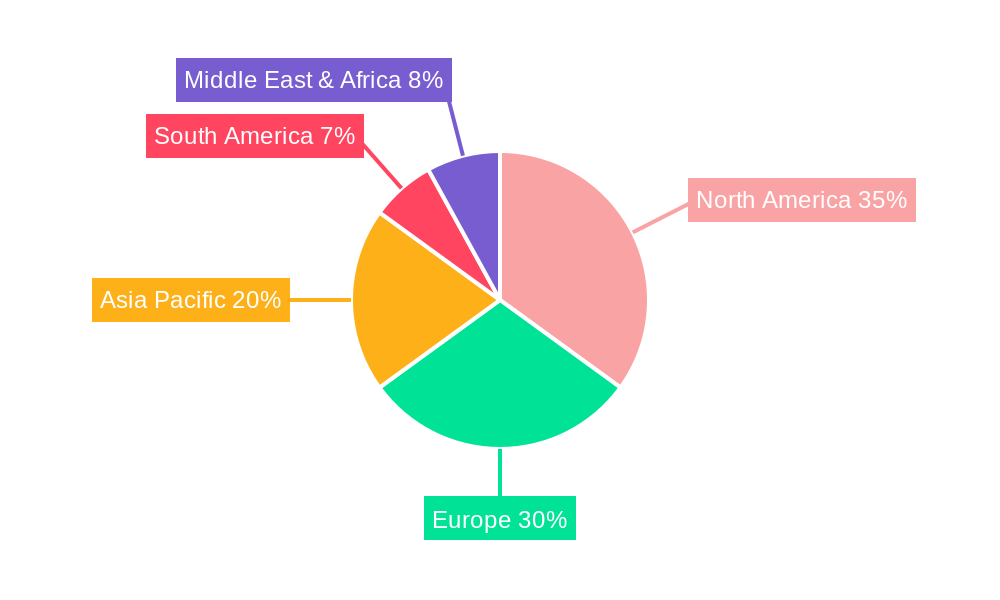
The Intramuscular Injection application segment currently dominates the Imipenem API market, accounting for an estimated 65% of the global market share in 2024. This dominance is driven by the established clinical protocols and efficacy of intramuscular administration for treating various severe systemic infections. The Aseptic API type also holds a significant share, estimated at 70% of the market, due to the critical need for sterile APIs in injectable formulations to prevent patient harm.
Dominant Application Segment: Intramuscular Injection
- Key Drivers: Efficacy in treating severe systemic infections, established treatment protocols, broad spectrum of activity.
- Market Share (2024): Approximately 65% of the Imipenem API market.
- Growth Potential: Continued demand driven by bacterial infection prevalence, especially in hospital settings.
Dominant API Type: Aseptic API
- Key Drivers: Requirement for sterile formulations in injectable drugs, patient safety, regulatory mandates.
- Market Share (2024): Approximately 70% of the Imipenem API market.
- Growth Potential: Steady demand linked to injectable pharmaceutical production.
North America, particularly the United States, represents the leading regional market for Imipenem API. This dominance is attributed to its advanced healthcare infrastructure, high expenditure on pharmaceuticals, and the significant burden of infectious diseases. Robust research and development activities, coupled with a strong regulatory framework that encourages innovation, further bolster its market leadership. Economic policies supporting pharmaceutical manufacturing and access to healthcare services contribute to sustained demand. The market share for North America in 2024 was estimated at 35%. Europe, with its well-established pharmaceutical industry and high prevalence of hospital-acquired infections, is the second-largest market.
Imipenem API Product Landscape
The Imipenem API product landscape is characterized by high-purity grades manufactured to stringent pharmaceutical standards. Innovations are primarily focused on optimizing synthesis pathways to improve yield, reduce impurities, and enhance cost-effectiveness. Key product applications revolve around the formulation of injectable drugs for treating a wide spectrum of Gram-positive and Gram-negative bacterial infections, including those caused by multidrug-resistant organisms. Performance metrics emphasize high purity levels (often exceeding 99%), consistent particle size distribution, and stability under specified storage conditions, all crucial for ensuring the efficacy and safety of the final pharmaceutical product. Unique selling propositions often lie in the robust quality control systems employed by manufacturers and their adherence to global regulatory guidelines.
Key Drivers, Barriers & Challenges in Imipenem API
Key Drivers:
- Rising Incidence of Bacterial Infections: Increasing prevalence of severe bacterial infections, including hospital-acquired infections (HAIs), fuels the demand for broad-spectrum antibiotics like Imipenem.
- Antibiotic Resistance: The growing threat of antibiotic-resistant bacteria necessitates the use of powerful antibiotics like Imipenem as a last resort.
- Technological Advancements in API Manufacturing: Innovations in synthesis and purification processes are leading to more efficient and cost-effective production of Imipenem API.
- Growth of the Pharmaceutical Industry: Expansion of the global pharmaceutical sector and increasing healthcare expenditure, particularly in emerging economies, drives demand.
Key Barriers & Challenges:
- Stringent Regulatory Requirements: Obtaining and maintaining regulatory approvals for API manufacturing is a complex and time-consuming process, creating a barrier to entry.
- Supply Chain Vulnerabilities: Global supply chain disruptions, geopolitical instability, and raw material price volatility can impact the availability and cost of Imipenem API.
- Intense Competition: The market faces competition from other broad-spectrum antibiotics and potential new drug discoveries, necessitating continuous innovation and cost optimization.
- Environmental Concerns: Manufacturing processes for APIs can have environmental impacts, requiring adherence to strict environmental regulations and sustainable practices. The estimated impact of supply chain disruptions on pricing can be as high as 15% in volatile periods.
Emerging Opportunities in Imipenem API
Emerging opportunities in the Imipenem API market lie in the development of novel formulations for improved drug delivery and patient compliance. Furthermore, untapped markets in developing regions with expanding healthcare infrastructure present significant growth potential. The increasing focus on combating neglected tropical diseases and emerging infectious diseases also opens avenues for Imipenem API utilization. Manufacturers can explore partnerships with research institutions to identify new therapeutic applications and improve existing treatment regimens. The growing demand for combination therapies that leverage Imipenem's broad spectrum of activity also presents an opportunity for API suppliers to tailor their products accordingly.
Growth Accelerators in the Imipenem API Industry
The Imipenem API industry is experiencing significant growth acceleration through several key catalysts. Technological breakthroughs in enzymatic synthesis and continuous manufacturing processes are enhancing production efficiency and reducing environmental impact. Strategic partnerships between API manufacturers and pharmaceutical formulators are streamlining the drug development pipeline and expediting market entry. Moreover, increasing global healthcare expenditure and a growing emphasis on infectious disease management in both developed and emerging economies are providing a robust demand foundation. The expansion of clinical research into new indications and the development of novel delivery systems for Imipenem are also acting as powerful growth accelerators, ensuring its continued relevance in combating bacterial infections.
Key Players Shaping the Imipenem API Market
- Hospira
- ACS DOBFAR SPA
- High Science
- Jeil Pharmaceutica
- Choongwae Pharma Corporation
- Sun Pharmaceutical
- Sandoz
- Kaliberr
- Auronext Pharma
- Nectar Lifesciences
- Savior Lifetec Corporation
- Zhuhai United Laboratorie
- SUZHOU HOMESUN PHARMACEUTICAL
- Unimark Remedies
- Zhejiang Haixiang Pharmaceutical
Notable Milestones in Imipenem API Sector
- 2019: Increased investment in research for novel synthesis routes to improve Imipenem API yield and purity.
- 2020: Heightened demand for broad-spectrum antibiotics due to global pandemic scenarios, boosting Imipenem API production.
- 2021: Introduction of advanced purification technologies by leading manufacturers to meet stringent quality standards for Imipenem API.
- 2022: Strategic collaborations formed between API suppliers and pharmaceutical companies to develop combination therapies involving Imipenem.
- 2023: Focus on supply chain resilience for critical APIs like Imipenem due to ongoing global logistical challenges.
- 2024: Expansion of manufacturing capacities by key players to meet anticipated future demand for Imipenem API.
In-Depth Imipenem API Market Outlook
The future outlook for the Imipenem API market remains exceptionally strong, propelled by persistent global health challenges. The ongoing rise in antibiotic resistance will continue to solidify Imipenem's position as a critical treatment option, driving sustained demand. Growth accelerators such as advancements in manufacturing technology, leading to more cost-effective and environmentally friendly production, will enhance market accessibility, particularly in emerging economies. Strategic alliances and continued investment in R&D for novel applications and improved formulations will further fortify the market. The Imipenem API market is poised for robust growth, offering significant strategic opportunities for stakeholders to capitalize on its essential role in modern medicine.
Imipenem API Segmentation
-
1. Application
- 1.1. Intramuscular Injection
- 1.2. Static Drops
-
2. Types
- 2.1. Aseptic API
- 2.2. Non-sterile API
Imipenem API Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. South America
- 2.1. Brazil
- 2.2. Argentina
- 2.3. Rest of South America
-
3. Europe
- 3.1. United Kingdom
- 3.2. Germany
- 3.3. France
- 3.4. Italy
- 3.5. Spain
- 3.6. Russia
- 3.7. Benelux
- 3.8. Nordics
- 3.9. Rest of Europe
-
4. Middle East & Africa
- 4.1. Turkey
- 4.2. Israel
- 4.3. GCC
- 4.4. North Africa
- 4.5. South Africa
- 4.6. Rest of Middle East & Africa
-
5. Asia Pacific
- 5.1. China
- 5.2. India
- 5.3. Japan
- 5.4. South Korea
- 5.5. ASEAN
- 5.6. Oceania
- 5.7. Rest of Asia Pacific
Imipenem API REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of XX% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.3. Market Restrains
- 3.4. Market Trends
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Imipenem API Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Application
- 5.1.1. Intramuscular Injection
- 5.1.2. Static Drops
- 5.2. Market Analysis, Insights and Forecast - by Types
- 5.2.1. Aseptic API
- 5.2.2. Non-sterile API
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. South America
- 5.3.3. Europe
- 5.3.4. Middle East & Africa
- 5.3.5. Asia Pacific
- 5.1. Market Analysis, Insights and Forecast - by Application
- 6. North America Imipenem API Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Application
- 6.1.1. Intramuscular Injection
- 6.1.2. Static Drops
- 6.2. Market Analysis, Insights and Forecast - by Types
- 6.2.1. Aseptic API
- 6.2.2. Non-sterile API
- 6.1. Market Analysis, Insights and Forecast - by Application
- 7. South America Imipenem API Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Application
- 7.1.1. Intramuscular Injection
- 7.1.2. Static Drops
- 7.2. Market Analysis, Insights and Forecast - by Types
- 7.2.1. Aseptic API
- 7.2.2. Non-sterile API
- 7.1. Market Analysis, Insights and Forecast - by Application
- 8. Europe Imipenem API Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Application
- 8.1.1. Intramuscular Injection
- 8.1.2. Static Drops
- 8.2. Market Analysis, Insights and Forecast - by Types
- 8.2.1. Aseptic API
- 8.2.2. Non-sterile API
- 8.1. Market Analysis, Insights and Forecast - by Application
- 9. Middle East & Africa Imipenem API Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Application
- 9.1.1. Intramuscular Injection
- 9.1.2. Static Drops
- 9.2. Market Analysis, Insights and Forecast - by Types
- 9.2.1. Aseptic API
- 9.2.2. Non-sterile API
- 9.1. Market Analysis, Insights and Forecast - by Application
- 10. Asia Pacific Imipenem API Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Application
- 10.1.1. Intramuscular Injection
- 10.1.2. Static Drops
- 10.2. Market Analysis, Insights and Forecast - by Types
- 10.2.1. Aseptic API
- 10.2.2. Non-sterile API
- 10.1. Market Analysis, Insights and Forecast - by Application
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2024
- 11.2. Company Profiles
- 11.2.1 Hospira
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 ACS DOBFAR SPA
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 High Science
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Jeil Pharmaceutica
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Choongwae Pharma Corporation
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Sun Pharmaceutical
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 Sandoz
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Kaliberr
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 Auronext Pharma
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Nectar Lifesciences
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 Savior Lifetec Corporation
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.12 Zhuhai United Laboratorie
- 11.2.12.1. Overview
- 11.2.12.2. Products
- 11.2.12.3. SWOT Analysis
- 11.2.12.4. Recent Developments
- 11.2.12.5. Financials (Based on Availability)
- 11.2.13 SUZHOU HOMESUN PHARMACEUTICAL
- 11.2.13.1. Overview
- 11.2.13.2. Products
- 11.2.13.3. SWOT Analysis
- 11.2.13.4. Recent Developments
- 11.2.13.5. Financials (Based on Availability)
- 11.2.14 Unimark Remedies
- 11.2.14.1. Overview
- 11.2.14.2. Products
- 11.2.14.3. SWOT Analysis
- 11.2.14.4. Recent Developments
- 11.2.14.5. Financials (Based on Availability)
- 11.2.15 Zhejiang Haixiang Pharmaceutical
- 11.2.15.1. Overview
- 11.2.15.2. Products
- 11.2.15.3. SWOT Analysis
- 11.2.15.4. Recent Developments
- 11.2.15.5. Financials (Based on Availability)
- 11.2.1 Hospira
List of Figures
- Figure 1: Global Imipenem API Revenue Breakdown (million, %) by Region 2024 & 2032
- Figure 2: North America Imipenem API Revenue (million), by Application 2024 & 2032
- Figure 3: North America Imipenem API Revenue Share (%), by Application 2024 & 2032
- Figure 4: North America Imipenem API Revenue (million), by Types 2024 & 2032
- Figure 5: North America Imipenem API Revenue Share (%), by Types 2024 & 2032
- Figure 6: North America Imipenem API Revenue (million), by Country 2024 & 2032
- Figure 7: North America Imipenem API Revenue Share (%), by Country 2024 & 2032
- Figure 8: South America Imipenem API Revenue (million), by Application 2024 & 2032
- Figure 9: South America Imipenem API Revenue Share (%), by Application 2024 & 2032
- Figure 10: South America Imipenem API Revenue (million), by Types 2024 & 2032
- Figure 11: South America Imipenem API Revenue Share (%), by Types 2024 & 2032
- Figure 12: South America Imipenem API Revenue (million), by Country 2024 & 2032
- Figure 13: South America Imipenem API Revenue Share (%), by Country 2024 & 2032
- Figure 14: Europe Imipenem API Revenue (million), by Application 2024 & 2032
- Figure 15: Europe Imipenem API Revenue Share (%), by Application 2024 & 2032
- Figure 16: Europe Imipenem API Revenue (million), by Types 2024 & 2032
- Figure 17: Europe Imipenem API Revenue Share (%), by Types 2024 & 2032
- Figure 18: Europe Imipenem API Revenue (million), by Country 2024 & 2032
- Figure 19: Europe Imipenem API Revenue Share (%), by Country 2024 & 2032
- Figure 20: Middle East & Africa Imipenem API Revenue (million), by Application 2024 & 2032
- Figure 21: Middle East & Africa Imipenem API Revenue Share (%), by Application 2024 & 2032
- Figure 22: Middle East & Africa Imipenem API Revenue (million), by Types 2024 & 2032
- Figure 23: Middle East & Africa Imipenem API Revenue Share (%), by Types 2024 & 2032
- Figure 24: Middle East & Africa Imipenem API Revenue (million), by Country 2024 & 2032
- Figure 25: Middle East & Africa Imipenem API Revenue Share (%), by Country 2024 & 2032
- Figure 26: Asia Pacific Imipenem API Revenue (million), by Application 2024 & 2032
- Figure 27: Asia Pacific Imipenem API Revenue Share (%), by Application 2024 & 2032
- Figure 28: Asia Pacific Imipenem API Revenue (million), by Types 2024 & 2032
- Figure 29: Asia Pacific Imipenem API Revenue Share (%), by Types 2024 & 2032
- Figure 30: Asia Pacific Imipenem API Revenue (million), by Country 2024 & 2032
- Figure 31: Asia Pacific Imipenem API Revenue Share (%), by Country 2024 & 2032
List of Tables
- Table 1: Global Imipenem API Revenue million Forecast, by Region 2019 & 2032
- Table 2: Global Imipenem API Revenue million Forecast, by Application 2019 & 2032
- Table 3: Global Imipenem API Revenue million Forecast, by Types 2019 & 2032
- Table 4: Global Imipenem API Revenue million Forecast, by Region 2019 & 2032
- Table 5: Global Imipenem API Revenue million Forecast, by Application 2019 & 2032
- Table 6: Global Imipenem API Revenue million Forecast, by Types 2019 & 2032
- Table 7: Global Imipenem API Revenue million Forecast, by Country 2019 & 2032
- Table 8: United States Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 9: Canada Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 10: Mexico Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 11: Global Imipenem API Revenue million Forecast, by Application 2019 & 2032
- Table 12: Global Imipenem API Revenue million Forecast, by Types 2019 & 2032
- Table 13: Global Imipenem API Revenue million Forecast, by Country 2019 & 2032
- Table 14: Brazil Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 15: Argentina Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 16: Rest of South America Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 17: Global Imipenem API Revenue million Forecast, by Application 2019 & 2032
- Table 18: Global Imipenem API Revenue million Forecast, by Types 2019 & 2032
- Table 19: Global Imipenem API Revenue million Forecast, by Country 2019 & 2032
- Table 20: United Kingdom Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 21: Germany Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 22: France Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 23: Italy Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 24: Spain Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 25: Russia Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 26: Benelux Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 27: Nordics Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 28: Rest of Europe Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 29: Global Imipenem API Revenue million Forecast, by Application 2019 & 2032
- Table 30: Global Imipenem API Revenue million Forecast, by Types 2019 & 2032
- Table 31: Global Imipenem API Revenue million Forecast, by Country 2019 & 2032
- Table 32: Turkey Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 33: Israel Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 34: GCC Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 35: North Africa Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 36: South Africa Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 37: Rest of Middle East & Africa Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 38: Global Imipenem API Revenue million Forecast, by Application 2019 & 2032
- Table 39: Global Imipenem API Revenue million Forecast, by Types 2019 & 2032
- Table 40: Global Imipenem API Revenue million Forecast, by Country 2019 & 2032
- Table 41: China Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 42: India Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 43: Japan Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 44: South Korea Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 45: ASEAN Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 46: Oceania Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
- Table 47: Rest of Asia Pacific Imipenem API Revenue (million) Forecast, by Application 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Imipenem API?
The projected CAGR is approximately XX%.
2. Which companies are prominent players in the Imipenem API?
Key companies in the market include Hospira, ACS DOBFAR SPA, High Science, Jeil Pharmaceutica, Choongwae Pharma Corporation, Sun Pharmaceutical, Sandoz, Kaliberr, Auronext Pharma, Nectar Lifesciences, Savior Lifetec Corporation, Zhuhai United Laboratorie, SUZHOU HOMESUN PHARMACEUTICAL, Unimark Remedies, Zhejiang Haixiang Pharmaceutical.
3. What are the main segments of the Imipenem API?
The market segments include Application, Types.
4. Can you provide details about the market size?
The market size is estimated to be USD XXX million as of 2022.
5. What are some drivers contributing to market growth?
N/A
6. What are the notable trends driving market growth?
N/A
7. Are there any restraints impacting market growth?
N/A
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 5600.00, USD 8400.00, and USD 11200.00 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Imipenem API," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Imipenem API report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Imipenem API?
To stay informed about further developments, trends, and reports in the Imipenem API, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


