Key Insights
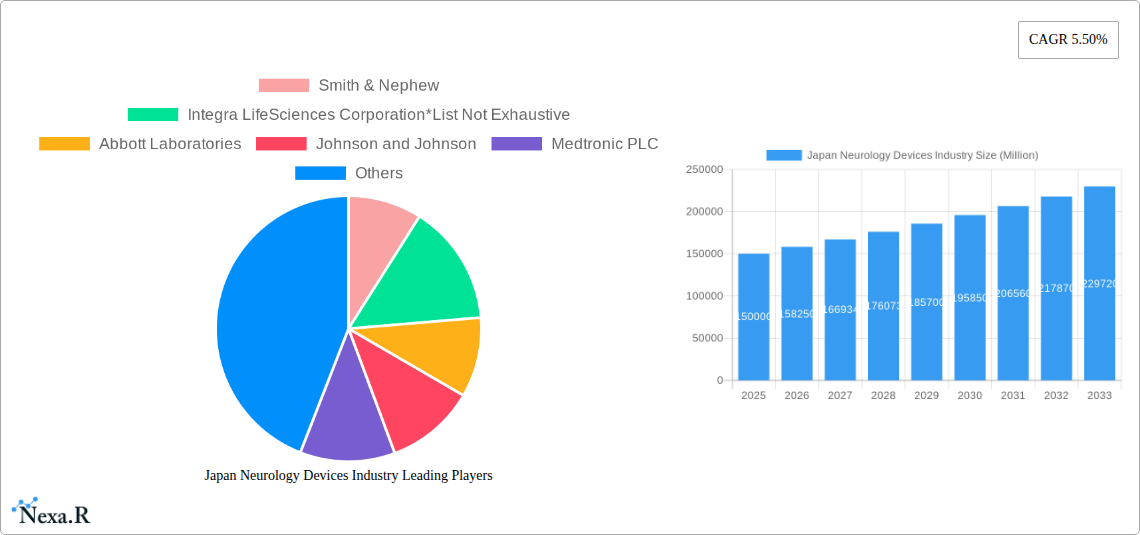
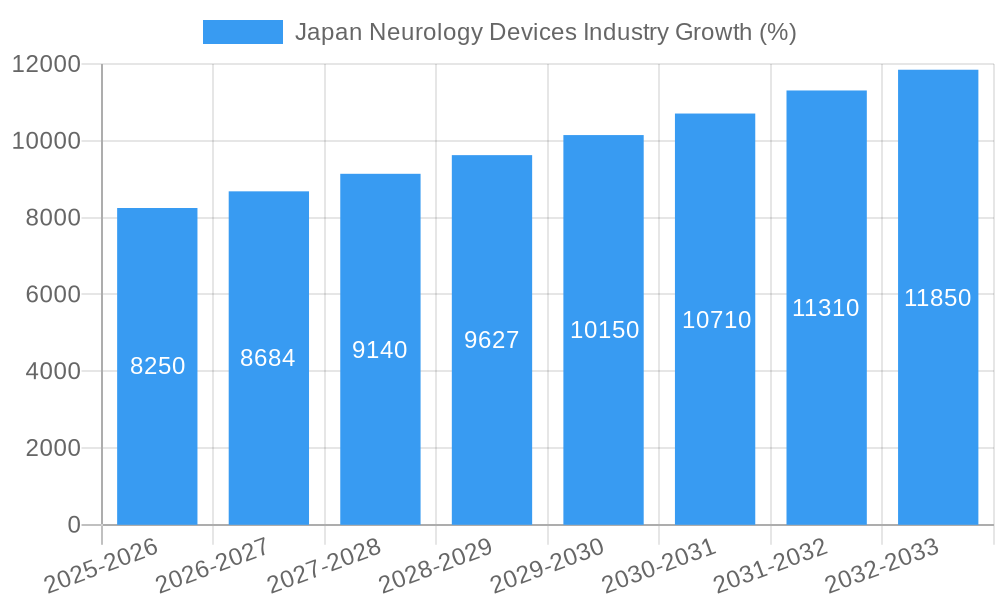
The Japan neurology devices market, valued at approximately ¥150 billion (assuming a market size of "XX" represents a comparable figure in Japanese Yen) in 2025, is projected to experience robust growth, driven by a rising geriatric population susceptible to neurological disorders like stroke and dementia. Technological advancements in minimally invasive procedures, sophisticated neurostimulation devices, and improved cerebrospinal fluid management systems are further fueling market expansion. The increasing prevalence of Alzheimer's disease and Parkinson's disease, coupled with growing healthcare expenditure and improved diagnostic capabilities, are key drivers. While the market faces potential restraints such as stringent regulatory approvals and high device costs, the strong emphasis on advanced medical technology within Japan's healthcare system is expected to mitigate these challenges. The segmentation by device type reflects a diverse market, with cerebrospinal fluid management devices and interventional neurology devices likely holding significant market share due to the increasing demand for minimally invasive procedures and advanced treatment options. Regional variations exist, with regions like Kanto and Kansai, possessing larger healthcare infrastructure and higher population densities, likely capturing a larger share of the market. The projected CAGR of 5.50% over the forecast period (2025-2033) suggests a considerable market expansion, reaching an estimated ¥250 billion by 2033.
The competitive landscape is marked by the presence of both established global players like Medtronic, Johnson & Johnson, and Boston Scientific, and regional players. These companies are engaged in intense R&D activities to enhance their product portfolio and gain a competitive advantage. Strategic partnerships, collaborations, and acquisitions are expected to shape the competitive dynamics in the coming years. The focus on innovation in areas like AI-powered diagnostic tools and personalized neurology treatments will be a major determinant of future market growth. The continuous development and adoption of advanced neurology devices, coupled with governmental initiatives aimed at improving healthcare infrastructure, position the Japan neurology devices market for sustained and significant growth throughout the forecast period. The market's success will hinge on the continuous advancement of technology, successful navigation of regulatory hurdles, and sustained investment in healthcare infrastructure.
Japan Neurology Devices Industry: Market Report 2019-2033
This comprehensive report provides an in-depth analysis of the Japan neurology devices market, encompassing market dynamics, growth trends, key players, and future outlook. The study period covers 2019-2033, with 2025 as the base and estimated year. This report is essential for industry professionals, investors, and strategic decision-makers seeking to understand and capitalize on opportunities within this dynamic sector. The report analyzes the parent market (Neurology Devices) and child markets (Cerebrospinal Fluid Management Devices, Interventional Neurology Devices, Neurosurgery Devices, Neurostimulation Devices, and Other Devices) to provide a holistic view. Market values are presented in million units.
Japan Neurology Devices Industry Market Dynamics & Structure
The Japan neurology devices market is characterized by a moderately concentrated landscape, with key players like Smith & Nephew, Integra LifeSciences Corporation, Abbott Laboratories, Johnson & Johnson, Medtronic PLC, Elekta AB, B. Braun Melsungen AG, Stryker Corporation, Boston Scientific Corporation, and Nihon Kohden Corporation holding significant market share. The market exhibits strong technological innovation, driven by advancements in minimally invasive procedures and neuro-stimulation technologies. Stringent regulatory frameworks, including those set by the Pharmaceuticals and Medical Devices Agency (PMDA), influence market access and product approvals. Competitive pressures arise from the availability of substitute therapies and the emergence of new entrants. The aging population in Japan significantly contributes to the increasing demand for neurology devices. The market has witnessed a moderate level of M&A activity in recent years, with xx deals recorded between 2019 and 2024, representing a xx% market share change.
- Market Concentration: Moderately concentrated, with top 10 players holding xx% market share in 2024.
- Technological Innovation: Driven by advancements in minimally invasive surgery, AI-powered diagnostics, and neurostimulation.
- Regulatory Framework: Stringent PMDA regulations influence market access and product approvals.
- Competitive Landscape: Intense competition among established players and emerging companies.
- M&A Activity: xx deals recorded between 2019 and 2024, resulting in a xx% shift in market share.
- Innovation Barriers: High R&D costs, stringent regulatory hurdles, and lengthy approval processes.
Japan Neurology Devices Industry Growth Trends & Insights
The Japan neurology devices market experienced a Compound Annual Growth Rate (CAGR) of xx% during the historical period (2019-2024), reaching a market size of xx million units in 2024. This growth is primarily attributed to the increasing prevalence of neurological disorders, an aging population, rising healthcare expenditure, and technological advancements leading to improved treatment options. The market is expected to continue its growth trajectory, with a projected CAGR of xx% during the forecast period (2025-2033), reaching xx million units by 2033. Increased adoption of minimally invasive procedures, the introduction of innovative devices, and government initiatives to improve healthcare infrastructure further fuel market expansion. Consumer behavior is shifting towards more personalized and technologically advanced solutions.
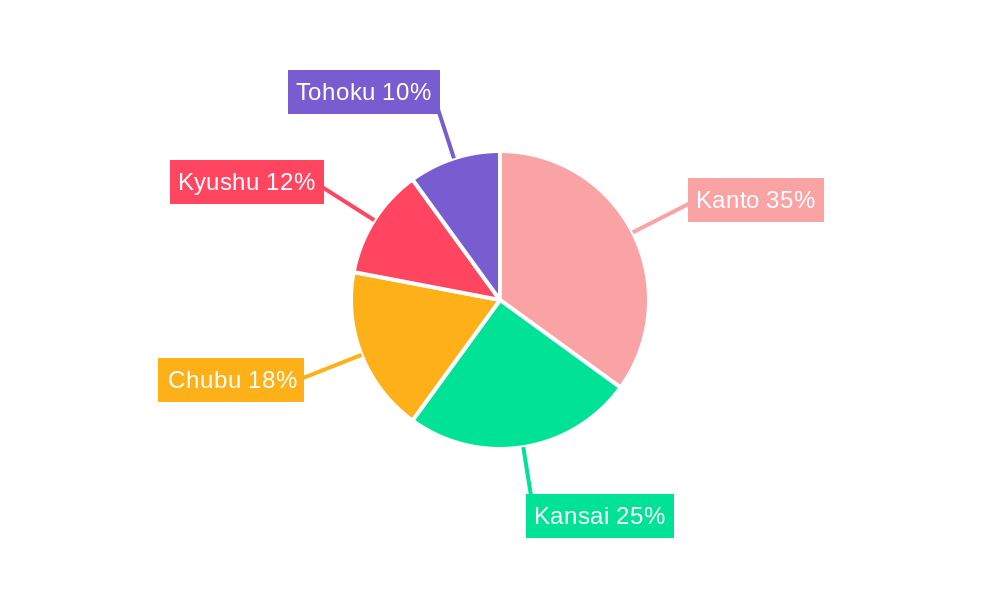
Dominant Regions, Countries, or Segments in Japan Neurology Devices Industry
The Kanto region, encompassing Tokyo and surrounding prefectures, dominates the Japan neurology devices market, accounting for approximately xx% of the total market share in 2024. This dominance stems from the high concentration of specialized hospitals, research institutions, and medical professionals in the area. Within the product segments, Interventional Neurology Devices currently holds the largest market share (xx%), followed by Neurosurgery Devices (xx%) and Cerebrospinal Fluid Management Devices (xx%). The growth of these segments is driven by factors such as:
- High Prevalence of Neurological Disorders: The rising incidence of stroke, Parkinson's disease, and Alzheimer's disease fuels demand.
- Technological Advancements: Minimally invasive procedures and advanced neuro-stimulation devices are gaining popularity.
- Government Initiatives: Increased healthcare expenditure and supportive policies drive market expansion.
Japan Neurology Devices Industry Product Landscape
The Japan neurology devices market offers a diverse range of products, including advanced cerebrospinal fluid management systems, sophisticated interventional neurology devices, and innovative neurostimulation technologies. These products feature improved safety profiles, enhanced functionalities, and minimized invasiveness. Key features include real-time monitoring capabilities, improved imaging technology, and enhanced ergonomics. This constant innovation drives market growth and ensures better patient outcomes.
Key Drivers, Barriers & Challenges in Japan Neurology Devices Industry
Key Drivers: The aging population, increased prevalence of neurological disorders, rising healthcare expenditure, and technological advancements are major drivers. Government initiatives to improve healthcare infrastructure also play a vital role.
Key Challenges: Stringent regulatory approvals, high R&D costs, and potential supply chain disruptions represent significant barriers. Competition from both established and emerging players, along with pricing pressures, also impacts market growth. The regulatory approval process can add an estimated xx months to product launch timelines, delaying revenue generation. Supply chain issues due to global events may cause material shortages and price increases.
Emerging Opportunities in Japan Neurology Devices Industry
Untapped opportunities lie in the development of personalized therapies, advanced diagnostic tools, and minimally invasive procedures. The rising demand for home healthcare solutions presents a new avenue for growth. Furthermore, the integration of AI and machine learning in neurology device design and application holds significant promise.
Growth Accelerators in the Japan Neurology Devices Industry
Strategic partnerships between domestic and international companies, coupled with investments in R&D and technological innovation, are crucial growth catalysts. Government initiatives promoting innovation and market access for novel neurology devices are further bolstering market expansion.
Key Players Shaping the Japan Neurology Devices Industry Market
- Smith & Nephew
- Integra LifeSciences Corporation
- Abbott Laboratories
- Johnson & Johnson
- Medtronic PLC
- Elekta AB
- B Braun Melsungen AG
- Stryker Corporation
- Boston Scientific Corporation
- Nihon Kohden Corporation
Notable Milestones in Japan Neurology Devices Industry Sector
- September 2022: Shionogi & Co., Ltd. and Pixie Dust Technologies, Inc. partnered to launch a brain activation business using sound stimulation, signifying a move towards novel therapeutic approaches.
- December 2020: Terumo Corporation launched the WEB Embolization System, expanding treatment options for brain aneurysms and potentially increasing market share.
In-Depth Japan Neurology Devices Industry Market Outlook
The Japan neurology devices market is poised for continued growth, driven by technological advancements, increasing prevalence of neurological diseases, and supportive government policies. Strategic investments in R&D, partnerships, and market expansion strategies will be crucial for companies to capitalize on this lucrative market's potential. The projected CAGR of xx% over the forecast period underscores significant opportunities for both established players and emerging companies.
Japan Neurology Devices Industry Segmentation
-
1. Type of Device
- 1.1. Cerebrospinal Fluid Management Devices
-
1.2. Interventional Neurology Devices
- 1.2.1. Interventional/Surgical Simulators
- 1.2.2. Neurothrombectomy Devices
- 1.2.3. Carotid Artery Stents
- 1.2.4. Others
-
1.3. Neurosurgery Devices
- 1.3.1. Neuroendoscopes
- 1.3.2. Stereotactic Systems
- 1.3.3. Other Neurosurgery Devices
-
1.4. Neurostimulation Devices
- 1.4.1. Spinal Cord Stimulation Devices
- 1.4.2. Deep Brain Stimulation Devices
- 1.4.3. Sacral Nerve Stimulation Devices
- 1.4.4. Other Neurostimulation Devices
- 1.5. Other Types of Devices
Japan Neurology Devices Industry Segmentation By Geography
- 1. Japan
Japan Neurology Devices Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 5.50% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increase in Incidence of Neurological Disorders; Huge Investments by Private Players in Neurology Devices; Increase in R&D in the Field of Neurotherapies
- 3.3. Market Restrains
- 3.3.1. High Cost of Equipment; Stringent FDA Validation and Guidelines for New Devices
- 3.4. Market Trends
- 3.4.1. Cerebrospinal Fluid Management Device is Expected to Hold Significant Share Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Japan Neurology Devices Industry Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Type of Device
- 5.1.1. Cerebrospinal Fluid Management Devices
- 5.1.2. Interventional Neurology Devices
- 5.1.2.1. Interventional/Surgical Simulators
- 5.1.2.2. Neurothrombectomy Devices
- 5.1.2.3. Carotid Artery Stents
- 5.1.2.4. Others
- 5.1.3. Neurosurgery Devices
- 5.1.3.1. Neuroendoscopes
- 5.1.3.2. Stereotactic Systems
- 5.1.3.3. Other Neurosurgery Devices
- 5.1.4. Neurostimulation Devices
- 5.1.4.1. Spinal Cord Stimulation Devices
- 5.1.4.2. Deep Brain Stimulation Devices
- 5.1.4.3. Sacral Nerve Stimulation Devices
- 5.1.4.4. Other Neurostimulation Devices
- 5.1.5. Other Types of Devices
- 5.2. Market Analysis, Insights and Forecast - by Region
- 5.2.1. Japan
- 5.1. Market Analysis, Insights and Forecast - by Type of Device
- 6. Kanto Japan Neurology Devices Industry Analysis, Insights and Forecast, 2019-2031
- 7. Kansai Japan Neurology Devices Industry Analysis, Insights and Forecast, 2019-2031
- 8. Chubu Japan Neurology Devices Industry Analysis, Insights and Forecast, 2019-2031
- 9. Kyushu Japan Neurology Devices Industry Analysis, Insights and Forecast, 2019-2031
- 10. Tohoku Japan Neurology Devices Industry Analysis, Insights and Forecast, 2019-2031
- 11. Competitive Analysis
- 11.1. Market Share Analysis 2024
- 11.2. Company Profiles
- 11.2.1 Smith & Nephew
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Integra LifeSciences Corporation*List Not Exhaustive
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 Abbott Laboratories
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Johnson and Johnson
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Medtronic PLC
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Elekta AB
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 B Braun Melsungen AG
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Stryker Corporation
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 Boston Scientific Corporation
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Nihon Kohden Corporation
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.1 Smith & Nephew
List of Figures
- Figure 1: Japan Neurology Devices Industry Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: Japan Neurology Devices Industry Share (%) by Company 2024
List of Tables
- Table 1: Japan Neurology Devices Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Japan Neurology Devices Industry Revenue Million Forecast, by Type of Device 2019 & 2032
- Table 3: Japan Neurology Devices Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 4: Japan Neurology Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 5: Kanto Japan Neurology Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 6: Kansai Japan Neurology Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 7: Chubu Japan Neurology Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 8: Kyushu Japan Neurology Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 9: Tohoku Japan Neurology Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 10: Japan Neurology Devices Industry Revenue Million Forecast, by Type of Device 2019 & 2032
- Table 11: Japan Neurology Devices Industry Revenue Million Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Japan Neurology Devices Industry?
The projected CAGR is approximately 5.50%.
2. Which companies are prominent players in the Japan Neurology Devices Industry?
Key companies in the market include Smith & Nephew, Integra LifeSciences Corporation*List Not Exhaustive, Abbott Laboratories, Johnson and Johnson, Medtronic PLC, Elekta AB, B Braun Melsungen AG, Stryker Corporation, Boston Scientific Corporation, Nihon Kohden Corporation.
3. What are the main segments of the Japan Neurology Devices Industry?
The market segments include Type of Device.
4. Can you provide details about the market size?
The market size is estimated to be USD XX Million as of 2022.
5. What are some drivers contributing to market growth?
Increase in Incidence of Neurological Disorders; Huge Investments by Private Players in Neurology Devices; Increase in R&D in the Field of Neurotherapies.
6. What are the notable trends driving market growth?
Cerebrospinal Fluid Management Device is Expected to Hold Significant Share Over the Forecast Period.
7. Are there any restraints impacting market growth?
High Cost of Equipment; Stringent FDA Validation and Guidelines for New Devices.
8. Can you provide examples of recent developments in the market?
September 2022: Shionogi & Co., Ltd. and Pixie Dust Technologies, Inc. announced that they have entered into a business partnership agreement (hereafter 'the Partnership Agreement') to launch a business involving brain activation through sound stimulation.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3800, USD 4500, and USD 5800 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Japan Neurology Devices Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Japan Neurology Devices Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Japan Neurology Devices Industry?
To stay informed about further developments, trends, and reports in the Japan Neurology Devices Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


