Key Insights
The Japan respiratory devices market, valued at approximately ¥300 billion (assuming a reasonable market size based on a mature market with a 9.60% CAGR) in 2025, is projected to experience robust growth throughout the forecast period (2025-2033). This expansion is fueled by several key drivers: an aging population with a rising prevalence of chronic respiratory diseases such as asthma, COPD, and sleep apnea; increasing healthcare expenditure; and technological advancements leading to the development of more sophisticated and minimally invasive respiratory devices. The market segments are dominated by diagnostic and monitoring devices, reflecting the importance of early detection and disease management. Therapeutic devices, including ventilators and oxygen therapy equipment, represent a significant portion as well, contributing substantially to the overall market value. Growth will be further propelled by government initiatives focused on improving healthcare access and quality, coupled with rising awareness among the population regarding respiratory health.
However, the market's growth trajectory is not without challenges. High costs associated with advanced respiratory devices can limit accessibility for certain patient segments. Furthermore, stringent regulatory approvals and reimbursement policies pose potential hurdles for market entrants. The competitive landscape is characterized by established international players alongside domestic manufacturers, resulting in a dynamic market environment. Regional variations in market penetration will likely persist, with regions like Kanto and Kansai, containing major urban centers and high healthcare infrastructure, leading the market share. The ongoing focus on developing portable and user-friendly devices, combined with telemedicine adoption, is poised to shape future market trends.

Japan Respiratory Devices Industry: 2019-2033 Market Report
This comprehensive report provides an in-depth analysis of the Japan respiratory devices industry, offering crucial insights for market participants, investors, and industry professionals. Covering the period 2019-2033, with a base year of 2025, this report dissects market dynamics, growth trends, and future opportunities within this vital sector. The report segments the market by Type: Diagnostic and Monitoring Devices, Other Diagnostic and Monitoring Devices, Therapeutic Devices, Other Therapeutic Devices, and Disposables, offering granular analysis of each segment's performance and growth trajectory. Key players such as CHEST M I Inc, Terumo Corporation, GE Healthcare, Medtronic PLC, Getinge AB, Metran Co Ltd, Koninklijke Philips NV, ResMed Inc, Dragerwerk AG, and Fisher & Paykel Healthcare Ltd are profiled, providing a complete picture of the competitive landscape.
Japan Respiratory Devices Industry Market Dynamics & Structure
The Japan respiratory devices market exhibits a moderately concentrated structure, with established multinational corporations and domestic players vying for market share. Technological innovation, primarily driven by advancements in digital health and connected devices, is a major growth catalyst. Stringent regulatory frameworks, including those set by the Pharmaceuticals and Medical Devices Agency (PMDA), influence product approvals and market entry strategies. The presence of substitute therapies, like traditional medication, and rising healthcare costs create both opportunities and challenges for industry growth. The demographic shift towards an aging population significantly fuels demand for respiratory devices. M&A activity within the sector remains moderate, with strategic acquisitions focused on enhancing technological capabilities and expanding product portfolios. Over the period of 2019 to 2024, we estimate around xx M&A deals with a total value of xx million USD.
- Market Concentration: Moderately Concentrated. Top 5 players hold approximately xx% market share.
- Technological Innovation: Focus on digital health integration, AI-driven diagnostics, and smart inhalers.
- Regulatory Landscape: Stringent PMDA approvals, impacting time-to-market for new products.
- Competitive Substitutes: Traditional medications and alternative therapies pose competitive pressure.
- End-User Demographics: Aging population drives demand for respiratory care solutions.
- M&A Trends: Strategic acquisitions focused on technology and product portfolio expansion.
Japan Respiratory Devices Industry Growth Trends & Insights
The Japan respiratory devices market has experienced a steady growth trajectory over the historical period (2019-2024), with the market size estimated at xx million units in 2024. This growth is attributed to increasing prevalence of respiratory illnesses like asthma and COPD, combined with rising awareness and improved healthcare infrastructure. Technological disruptions, such as the introduction of smart inhalers and remote patient monitoring systems, are accelerating market adoption. Changing consumer behavior, favoring convenient and technologically advanced solutions, further fuels this growth. The projected Compound Annual Growth Rate (CAGR) for the forecast period (2025-2033) is estimated at xx%, with the market size reaching xx million units by 2033. Market penetration is expected to increase from xx% in 2024 to xx% by 2033, driven primarily by increasing affordability and improved accessibility of respiratory devices.
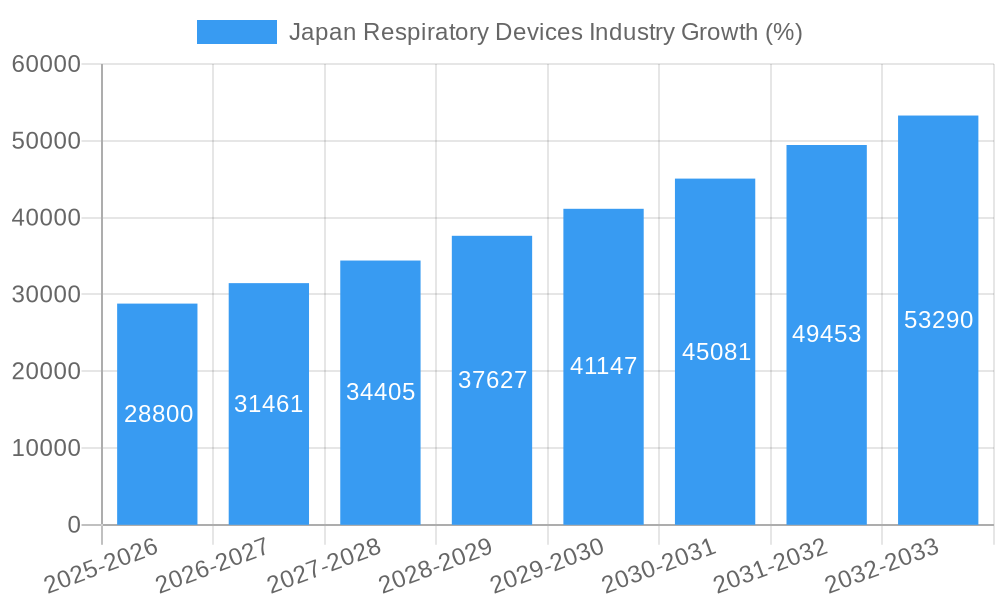
Dominant Regions, Countries, or Segments in Japan Respiratory Devices Industry
The Kanto region, encompassing Tokyo and surrounding prefectures, represents the dominant market segment, owing to its dense population, advanced healthcare infrastructure, and high concentration of medical institutions. Within the product segments, Diagnostic and Monitoring Devices, particularly those incorporating digital health features, demonstrate robust growth potential. This is primarily driven by increasing government funding for healthcare technology, early disease detection initiatives, and a rising awareness amongst consumers about the value of proactive health management. The Therapeutic Devices segment also holds significant growth potential with the increased adoption of innovative drug delivery systems.
- Key Drivers (Kanto Region): High population density, advanced healthcare infrastructure, concentration of medical facilities.
- Key Drivers (Diagnostic and Monitoring Devices): Government funding for healthcare technology, early disease detection initiatives, rising consumer awareness.
- Key Drivers (Therapeutic Devices): Increased adoption of innovative drug delivery systems.
- Market Share: Kanto region holds approximately xx% of the total market share.
- Growth Potential: Diagnostic and Monitoring Devices are projected to exhibit the highest CAGR over the forecast period.
Japan Respiratory Devices Industry Product Landscape
The Japanese market showcases a diverse range of respiratory devices, encompassing advanced diagnostic tools, sophisticated therapeutic systems, and convenient disposables. Product innovations focus on improved user-friendliness, enhanced data connectivity, and miniature device designs for improved patient compliance. These advancements enhance treatment efficacy and data monitoring capabilities. Technological advancements such as smart inhalers, incorporating sensors and data logging capabilities, alongside nebulizers offering improved drug delivery, are shaping the product landscape. The unique selling propositions often include enhanced ease of use, improved accuracy in diagnosis, and efficient data integration with electronic health records.
Key Drivers, Barriers & Challenges in Japan Respiratory Devices Industry
Key Drivers:
- Increasing prevalence of chronic respiratory diseases like asthma and COPD.
- Growing government initiatives and investments in healthcare infrastructure and technology.
- Rising consumer awareness regarding respiratory health and self-management.
- Technological advancements, leading to the introduction of innovative devices and therapies.
Key Challenges:
- High regulatory hurdles and stringent approval processes for new devices.
- High cost of devices, hindering accessibility for certain demographics.
- Intense competition from both domestic and international players.
- Supply chain vulnerabilities and potential disruptions impacting device availability. The impact of xx disruptions in 2022 resulted in a xx% reduction in device supply.
Emerging Opportunities in Japan Respiratory Devices Industry
Emerging opportunities include the integration of artificial intelligence (AI) in diagnostic and therapeutic devices, personalized medicine approaches to respiratory care, and the expansion into remote patient monitoring systems for improved patient outcomes. Untapped markets include rural areas with limited access to healthcare facilities, while the evolving consumer preference for home-based respiratory care solutions presents another promising avenue for growth.
Growth Accelerators in the Japan Respiratory Devices Industry
Long-term growth will be fueled by technological breakthroughs in areas such as AI-driven diagnostics, minimally invasive therapies, and personalized medicine. Strategic partnerships between device manufacturers, pharmaceutical companies, and healthcare providers will play a crucial role in market expansion. Government initiatives aimed at improving healthcare accessibility and affordability will also act as a key growth accelerator. Furthermore, the increasing adoption of telehealth solutions and the development of sophisticated remote patient monitoring systems will drive long-term growth.
Key Players Shaping the Japan Respiratory Devices Industry Market
- CHEST M I Inc
- Terumo Corporation
- GE Healthcare
- Medtronic PLC
- Getinge AB
- Metran Co Ltd
- Koninklijke Philips NV
- ResMed Inc
- Dragerwerk AG
- Fisher & Paykel Healthcare Ltd
Notable Milestones in Japan Respiratory Devices Industry Sector
- Feb 2022: Aptar Pharma launched HeroTracker Sense, a smart inhaler, enhancing digital respiratory health solutions. This launch significantly impacted the market by introducing a connected device into the space, potentially influencing patient compliance and data-driven care.
- Mar 2021: PARI Pharma GmbH received authorization for the LAMIRA Nebulizer System for ARIKAYCE delivery, expanding treatment options for specific respiratory illnesses. This authorization opened a new market segment for specialized drug delivery solutions.
In-Depth Japan Respiratory Devices Industry Market Outlook
The Japan respiratory devices market holds significant future potential driven by an aging population, increasing prevalence of respiratory diseases, and continuous technological innovation. Strategic partnerships, focused on integrating digital health technologies and expanding into underserved markets, will be crucial for sustained growth. The focus on preventative care, proactive health management, and the development of personalized treatment strategies will continue to shape the market trajectory. The market is poised to witness robust growth, driven by the factors mentioned above, and presents significant opportunities for market players who can successfully navigate the regulatory landscape and adapt to evolving consumer preferences.
Japan Respiratory Devices Industry Segmentation
-
1. Type
-
1.1. Diagnostic and Monitoring Devices
- 1.1.1. Spirometers
- 1.1.2. Sleep Test Devices
- 1.1.3. Peak Flow Meters
- 1.1.4. Other Diagnostic and Monitoring Devices
-
1.2. Therapeutic Devices
- 1.2.1. Ventilators
- 1.2.2. Inhalers
- 1.2.3. CPAP Devices
- 1.2.4. Oxygen Concentrators
- 1.2.5. Other Therapeutic Devices
-
1.3. Disposables
- 1.3.1. Masks
- 1.3.2. Breathing Circuits
- 1.3.3. Other Disposables
-
1.1. Diagnostic and Monitoring Devices
Japan Respiratory Devices Industry Segmentation By Geography
- 1. Japan

Japan Respiratory Devices Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 9.60% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Prevalence of Respiratory Disorders; Technological Advancements in the Devices
- 3.3. Market Restrains
- 3.3.1. High Cost of Equipment
- 3.4. Market Trends
- 3.4.1. Inhalers Segment is Expected to Witness Strong Growth Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Japan Respiratory Devices Industry Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Type
- 5.1.1. Diagnostic and Monitoring Devices
- 5.1.1.1. Spirometers
- 5.1.1.2. Sleep Test Devices
- 5.1.1.3. Peak Flow Meters
- 5.1.1.4. Other Diagnostic and Monitoring Devices
- 5.1.2. Therapeutic Devices
- 5.1.2.1. Ventilators
- 5.1.2.2. Inhalers
- 5.1.2.3. CPAP Devices
- 5.1.2.4. Oxygen Concentrators
- 5.1.2.5. Other Therapeutic Devices
- 5.1.3. Disposables
- 5.1.3.1. Masks
- 5.1.3.2. Breathing Circuits
- 5.1.3.3. Other Disposables
- 5.1.1. Diagnostic and Monitoring Devices
- 5.2. Market Analysis, Insights and Forecast - by Region
- 5.2.1. Japan
- 5.1. Market Analysis, Insights and Forecast - by Type
- 6. Kanto Japan Respiratory Devices Industry Analysis, Insights and Forecast, 2019-2031
- 7. Kansai Japan Respiratory Devices Industry Analysis, Insights and Forecast, 2019-2031
- 8. Chubu Japan Respiratory Devices Industry Analysis, Insights and Forecast, 2019-2031
- 9. Kyushu Japan Respiratory Devices Industry Analysis, Insights and Forecast, 2019-2031
- 10. Tohoku Japan Respiratory Devices Industry Analysis, Insights and Forecast, 2019-2031
- 11. Competitive Analysis
- 11.1. Market Share Analysis 2024
- 11.2. Company Profiles
- 11.2.1 CHEST M I Inc
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Terumo Corporation
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 GE Healthcare
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Medtronic PLC
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Getinge AB
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Metran Co Ltd*List Not Exhaustive
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 Koninklijke Philips NV
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 ResMed Inc
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 Dragerwerk AG
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Fisher & Paykel Healthcare Ltd
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.1 CHEST M I Inc
List of Figures
- Figure 1: Japan Respiratory Devices Industry Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: Japan Respiratory Devices Industry Share (%) by Company 2024
List of Tables
- Table 1: Japan Respiratory Devices Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Japan Respiratory Devices Industry Revenue Million Forecast, by Type 2019 & 2032
- Table 3: Japan Respiratory Devices Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 4: Japan Respiratory Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 5: Kanto Japan Respiratory Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 6: Kansai Japan Respiratory Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 7: Chubu Japan Respiratory Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 8: Kyushu Japan Respiratory Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 9: Tohoku Japan Respiratory Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 10: Japan Respiratory Devices Industry Revenue Million Forecast, by Type 2019 & 2032
- Table 11: Japan Respiratory Devices Industry Revenue Million Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Japan Respiratory Devices Industry?
The projected CAGR is approximately 9.60%.
2. Which companies are prominent players in the Japan Respiratory Devices Industry?
Key companies in the market include CHEST M I Inc, Terumo Corporation, GE Healthcare, Medtronic PLC, Getinge AB, Metran Co Ltd*List Not Exhaustive, Koninklijke Philips NV, ResMed Inc, Dragerwerk AG, Fisher & Paykel Healthcare Ltd.
3. What are the main segments of the Japan Respiratory Devices Industry?
The market segments include Type.
4. Can you provide details about the market size?
The market size is estimated to be USD XX Million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Prevalence of Respiratory Disorders; Technological Advancements in the Devices.
6. What are the notable trends driving market growth?
Inhalers Segment is Expected to Witness Strong Growth Over the Forecast Period.
7. Are there any restraints impacting market growth?
High Cost of Equipment.
8. Can you provide examples of recent developments in the market?
Feb 2022: Aptar Pharma announced the launch of HeroTracker Sense, a novel digital respiratory health solution that transforms a standard metered dose inhaler (pMDI) into a smart, connected healthcare device.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3800, USD 4500, and USD 5800 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Japan Respiratory Devices Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Japan Respiratory Devices Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Japan Respiratory Devices Industry?
To stay informed about further developments, trends, and reports in the Japan Respiratory Devices Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


