Key Insights
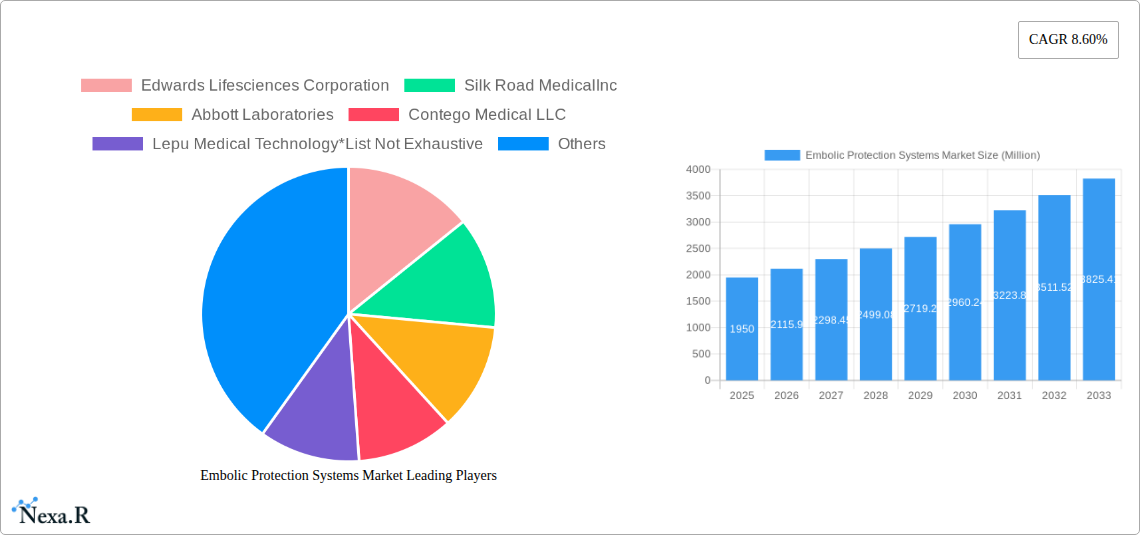
The global Embolic Protection Systems (EPS) market is poised for substantial growth, projected to reach an estimated USD 1,950 million by 2025, with a robust Compound Annual Growth Rate (CAGR) of 8.60% through 2033. This expansion is primarily fueled by the increasing prevalence of cardiovascular and neurovascular diseases, which necessitate advanced preventative measures against embolic events during interventional procedures. The rising demand for minimally invasive surgical techniques further propels the adoption of EPS devices, offering enhanced patient outcomes and reduced recovery times. Technological advancements in device design, materials science such as the utilization of advanced Nitinol and biocompatible Polyurethane, and a growing focus on patient safety are also significant drivers. The market is segmented into Distal Occlusion Devices, Distal Filters, and Proximal Occlusion Devices, with Nitinol and Polyurethane dominating material choices. Cardiovascular diseases represent the largest application segment, followed closely by neurovascular diseases.
The Embolic Protection Systems market is experiencing dynamic shifts driven by both innovation and evolving healthcare landscapes. Key players like Abbott Laboratories, Medtronic Plc, and Boston Scientific Corporation are at the forefront, investing heavily in research and development to introduce next-generation EPS solutions. While market growth is strong, certain restraints, such as the high cost of advanced EPS devices and the need for specialized training for interventional cardiologists and neurosurgeons, may temper the pace of adoption in some regions. However, the increasing awareness among healthcare providers and patients regarding the benefits of embolic protection, coupled with favorable reimbursement policies in developed economies, are expected to mitigate these challenges. Emerging economies, particularly in the Asia Pacific region, are presenting significant growth opportunities due to expanding healthcare infrastructure and a growing burden of cardiovascular and cerebrovascular diseases. This dynamic interplay of drivers and restraints, coupled with strategic company initiatives, will shape the trajectory of the EPS market in the coming years, reinforcing its critical role in patient care.
Embolic Protection Systems Market: Comprehensive Report & Forecast (2019-2033)
Unlock critical insights into the global Embolic Protection Systems (EPS) market. This definitive report provides an in-depth analysis of market dynamics, growth trends, regional dominance, product landscape, key drivers, emerging opportunities, and the competitive ecosystem. Essential for stakeholders seeking to navigate and capitalize on the evolving landscape of neurovascular and cardiovascular interventions.
Embolic Protection Systems Market Market Dynamics & Structure
The global Embolic Protection Systems market is characterized by a moderate to high concentration, driven by significant technological advancements and a growing awareness of stroke prevention during interventional procedures. Key drivers include the increasing prevalence of cardiovascular and neurovascular diseases, coupled with a rising demand for minimally invasive treatments. Regulatory frameworks, while crucial for ensuring patient safety and device efficacy, also present barriers to entry for new players, fostering a competitive environment among established manufacturers. The market experiences a constant push for innovation, with companies investing heavily in developing more effective and user-friendly EPS devices. Competitive product substitutes, such as advanced surgical techniques, exist but are increasingly being overshadowed by the benefits of percutaneous interventions enabled by EPS. End-user demographics are shifting towards an aging global population, which naturally exhibits a higher incidence of conditions requiring these interventions. Mergers and acquisitions (M&A) are an active component of market structure, allowing larger companies to expand their portfolios and market reach. For instance, historical M&A activity has seen consolidation in segments like cardiovascular devices, impacting the EPS landscape. Innovation barriers include the rigorous clinical trial requirements and the need for extensive product validation to gain regulatory approval.
- Market Concentration: Moderate to High
- Key Drivers: Rising prevalence of cardiovascular/neurovascular diseases, minimally invasive treatment demand.
- Regulatory Influence: Strict approvals, impacting new entrants.
- Competitive Landscape: Driven by innovation and strategic partnerships.
- M&A Activity: Significant for portfolio expansion and market consolidation.
- Innovation Barriers: High cost and complexity of clinical trials.
Embolic Protection Systems Market Growth Trends & Insights
The Embolic Protection Systems market is poised for robust expansion, fueled by a confluence of factors that are reshaping the healthcare sector. The market size is projected to witness a substantial Compound Annual Growth Rate (CAGR) of approximately 9.5% from 2025 to 2033, reaching an estimated value of USD 2,500 Million units by 2033. This growth trajectory is underpinned by increasing adoption rates of transcatheter procedures, such as transcatheter aortic valve replacement (TAVR) and transcatheter mitral valve repair (TMVR), which necessitate the use of EPS to mitigate embolic events. Technological disruptions are a constant theme, with ongoing research and development focused on creating smaller, more deliverable, and more effective embolic filters and occluders. Consumer behavior shifts are also playing a critical role, as patients and healthcare providers alike increasingly favor less invasive treatment options that offer faster recovery times and reduced hospital stays. The growing awareness of stroke prevention strategies during cardiovascular interventions is further propelling demand. Market penetration is expected to deepen as regulatory approvals expand to new geographies and as the cost-effectiveness of EPS in preventing costly neurological complications becomes more evident. The interplay between these trends suggests a dynamic and rapidly evolving market landscape, with significant opportunities for innovation and market leadership.
- Projected Market Size (2033): USD 2,500 Million units
- Estimated CAGR (2025-2033): ~9.5%
- Key Growth Drivers: Increased TAVR/TMVR procedures, technological advancements in EPS, growing awareness of stroke prevention, patient preference for minimally invasive treatments.
- Market Penetration: Expected to increase with expanded regulatory approvals and improved cost-effectiveness.
- Technological Disruptions: Focus on miniaturization, enhanced deliverability, and improved embolic capture efficiency.
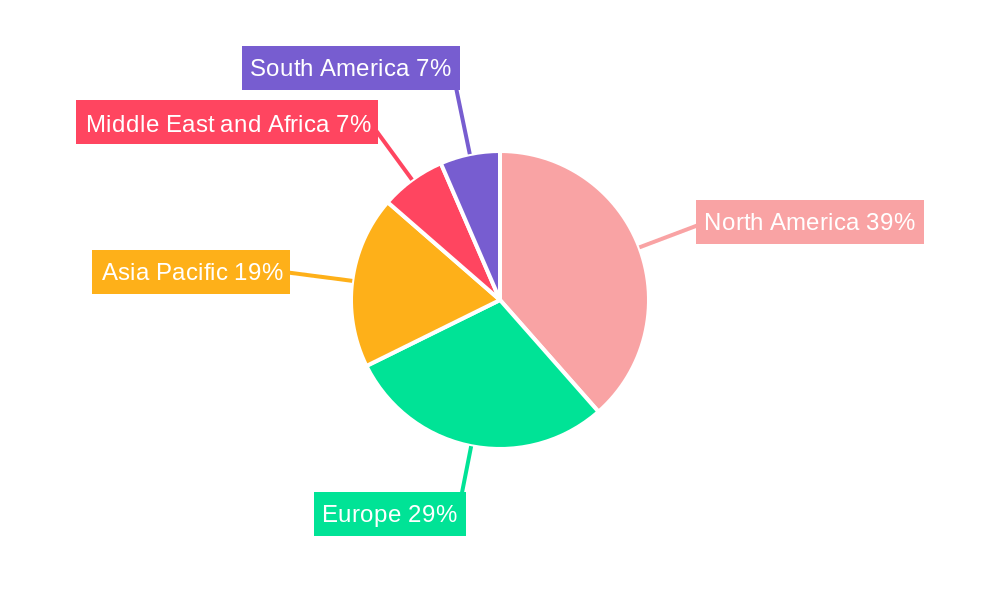
Dominant Regions, Countries, or Segments in Embolic Protection Systems Market
North America currently commands a significant share of the Embolic Protection Systems market, driven by a confluence of factors including a high prevalence of cardiovascular and neurovascular diseases, advanced healthcare infrastructure, and strong adoption rates of interventional cardiology and neurology procedures. The United States, in particular, stands as a dominant country within this region, characterized by robust reimbursement policies, a high disposable income for advanced medical treatments, and the presence of leading medical device manufacturers. The aging population in North America further contributes to the demand for EPS, as elderly individuals are more susceptible to stroke and other embolic complications.
Within the Product Technology Type segment, Distal Filters have historically dominated the market due to their widespread application in capturing embolic debris during various vascular interventions. However, Distal Occlusion Devices are experiencing rapid growth, driven by their enhanced ability to provide a more comprehensive embolic blockade in specific procedures.
The Material segment sees Nitinol as the leading choice, owing to its excellent biocompatibility, superelasticity, and radiopacity, making it ideal for sophisticated device designs that can navigate complex vasculature. Polyurethane also holds a significant share, particularly in device coatings and components.
In terms of Application, Cardiovascular Diseases represent the largest segment, propelled by the soaring number of percutaneous coronary interventions (PCI), TAVR, and other structural heart interventions. The increasing incidence of stroke and transient ischemic attacks (TIAs) also fuels the demand for EPS in Neurovascular Diseases.
- Dominant Region: North America
- Dominant Country: United States
- Leading Product Technology Type: Distal Filters (with strong growth in Distal Occlusion Devices)
- Leading Material: Nitinol
- Largest Application Segment: Cardiovascular Diseases
Embolic Protection Systems Market Product Landscape
The Embolic Protection Systems market is characterized by a landscape of continuous product innovation focused on enhancing embolic capture efficiency, ease of deployment, and patient safety. Manufacturers are investing in next-generation devices that feature finer mesh structures for superior filtration, advanced guidewire compatibility for improved deliverability through tortuous anatomies, and innovative retrieval mechanisms to minimize procedural risks. Unique selling propositions often lie in the proprietary design of the filter basket, its ability to conform to vessel walls, and its low profile for navigation. Technological advancements are also leading to the development of integrated systems that combine embolic protection with therapeutic delivery, streamlining complex interventional procedures.
Key Drivers, Barriers & Challenges in Embolic Protection Systems Market
Key Drivers:
- Increasing incidence of cardiovascular and neurovascular diseases: A growing global burden of these conditions directly translates to higher demand for interventional procedures, necessitating EPS.
- Advancements in minimally invasive cardiac and neurological procedures: The shift towards less invasive treatments like TAVR and stroke interventions significantly boosts EPS adoption.
- Technological innovation in device design: Development of more effective, user-friendly, and safer embolic protection devices.
- Growing physician and patient awareness: Increased understanding of the risks of embolic events and the benefits of prevention.
- Favorable reimbursement policies: Adequate coverage for interventional procedures and the use of EPS in various healthcare systems.
Barriers & Challenges:
- High cost of devices and procedures: Can limit accessibility in cost-sensitive healthcare markets.
- Stringent regulatory approval processes: Rigorous clinical trials and lengthy approval timelines can delay market entry for new products.
- Learning curve for physicians: Ensuring adequate training and proficiency in the use of complex EPS devices.
- Competition from alternative treatment modalities: While declining, surgical options remain a consideration for certain patient populations.
- Supply chain disruptions: Potential for interruptions in the availability of raw materials and finished goods, impacting market stability.
Emerging Opportunities in Embolic Protection Systems Market
Emerging opportunities in the Embolic Protection Systems market lie in the expansion into underdeveloped and emerging economies where the prevalence of cardiovascular and neurovascular diseases is rising, but access to advanced interventional technologies is limited. Furthermore, the development of next-generation EPS designed for highly specific and complex neurovascular interventions, such as those targeting posterior circulation strokes or complex intracranial aneurysms, presents a significant growth avenue. The integration of AI and advanced imaging with EPS devices to provide real-time guidance and efficacy monitoring during procedures is another promising frontier. Untapped applications in non-cardiac/neurovascular interventional procedures where embolic protection is beneficial also represent nascent opportunities. Evolving consumer preferences for personalized medicine and preventative healthcare could also drive demand for tailored EPS solutions.
Growth Accelerators in the Embolic Protection Systems Market Industry
Long-term growth in the Embolic Protection Systems market will be significantly accelerated by continuous technological breakthroughs, such as the development of bioresorbable embolic protection devices and miniaturized systems that can access smaller, more peripheral vessels. Strategic partnerships between device manufacturers and academic research institutions will foster the development of novel concepts and clinical validation, driving innovation. Market expansion strategies, including geographical diversification into Asia-Pacific and Latin America, where healthcare spending is on the rise, will be crucial. Moreover, the increasing emphasis on outcome-based healthcare and the reduction of stroke-related morbidities will incentivize the adoption of EPS as a standard of care, further accelerating market growth.
Key Players Shaping the Embolic Protection Systems Market Market
- Edwards Lifesciences Corporation
- Silk Road Medical Inc.
- Abbott Laboratories
- Contego Medical LLC
- Lepu Medical Technology
- Cardinal Health
- Hellman & Friedman LLC (Cordis)
- Medtronic Plc
- Boston Scientific Corporation
Notable Milestones in Embolic Protection Systems Market Sector
- November 2022: Innovative Cardiovascular Solutions sponsored a clinical study to evaluate the safety, effectiveness, and performance of the EMBLOK embolic protection systems (EPS) during transcatheter aortic valve replacement (TAVR) by randomized comparison with a commercially available embolic protection device.
- June 2022: Filterlex Medical publicized results from a first-in-human (FIH) study demonstrating the safety, feasibility, and performance of the Captis device, a full-body embolic protection device that reduces the risk of stroke and other complications during left-heart procedures.
In-Depth Embolic Protection Systems Market Market Outlook
The future outlook for the Embolic Protection Systems market is exceptionally promising, driven by a sustained increase in the demand for minimally invasive procedures and a heightened focus on patient safety. Technological advancements will continue to introduce more sophisticated devices with enhanced efficacy and usability, particularly for complex anatomical challenges in both neurovascular and cardiovascular interventions. Strategic collaborations, further clinical validation, and expanding reimbursement coverage across diverse healthcare systems will act as significant growth accelerators. The market's potential is further amplified by the growing burden of age-related cardiovascular and neurological conditions globally, creating a sustained need for effective embolic protection solutions. Stakeholders can anticipate a dynamic market characterized by innovation, strategic expansion, and a significant positive impact on patient outcomes.
Embolic Protection Systems Market Segmentation
-
1. Product Technology Type
- 1.1. Distal Occlusion Devices
- 1.2. Distal Filters
- 1.3. Proximal Occlusion Devices
-
2. Material
- 2.1. Nitinol
- 2.2. Polyurethane
-
3. Application
- 3.1. Neurovascular Diseases
- 3.2. Cardiovascular diseases
- 3.3. Others
Embolic Protection Systems Market Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. Europe
- 2.1. Germany
- 2.2. United Kingdom
- 2.3. France
- 2.4. Italy
- 2.5. Spain
- 2.6. Rest of Europe
-
3. Asia Pacific
- 3.1. China
- 3.2. Japan
- 3.3. India
- 3.4. Australia
- 3.5. South Korea
- 3.6. Rest of Asia Pacific
-
4. Middle East and Africa
- 4.1. GCC
- 4.2. South Africa
- 4.3. Rest of Middle East and Africa
-
5. South America
- 5.1. Brazil
- 5.2. Argentina
- 5.3. Rest of South America
Embolic Protection Systems Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 8.60% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Rise in Prevalence of Cardiovascular and Neurovascular Diseases; Increasing Demand for Minimally Invasive Surgeries
- 3.3. Market Restrains
- 3.3.1. Stringent Regulatory Scenarios and Unfavorable Government Policies; Lack of Skilled Labor
- 3.4. Market Trends
- 3.4.1. Cardiovascular Disease Segment is Expected to Witness a Notable Growth Over the Forecast period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Embolic Protection Systems Market Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Product Technology Type
- 5.1.1. Distal Occlusion Devices
- 5.1.2. Distal Filters
- 5.1.3. Proximal Occlusion Devices
- 5.2. Market Analysis, Insights and Forecast - by Material
- 5.2.1. Nitinol
- 5.2.2. Polyurethane
- 5.3. Market Analysis, Insights and Forecast - by Application
- 5.3.1. Neurovascular Diseases
- 5.3.2. Cardiovascular diseases
- 5.3.3. Others
- 5.4. Market Analysis, Insights and Forecast - by Region
- 5.4.1. North America
- 5.4.2. Europe
- 5.4.3. Asia Pacific
- 5.4.4. Middle East and Africa
- 5.4.5. South America
- 5.1. Market Analysis, Insights and Forecast - by Product Technology Type
- 6. North America Embolic Protection Systems Market Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Product Technology Type
- 6.1.1. Distal Occlusion Devices
- 6.1.2. Distal Filters
- 6.1.3. Proximal Occlusion Devices
- 6.2. Market Analysis, Insights and Forecast - by Material
- 6.2.1. Nitinol
- 6.2.2. Polyurethane
- 6.3. Market Analysis, Insights and Forecast - by Application
- 6.3.1. Neurovascular Diseases
- 6.3.2. Cardiovascular diseases
- 6.3.3. Others
- 6.1. Market Analysis, Insights and Forecast - by Product Technology Type
- 7. Europe Embolic Protection Systems Market Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Product Technology Type
- 7.1.1. Distal Occlusion Devices
- 7.1.2. Distal Filters
- 7.1.3. Proximal Occlusion Devices
- 7.2. Market Analysis, Insights and Forecast - by Material
- 7.2.1. Nitinol
- 7.2.2. Polyurethane
- 7.3. Market Analysis, Insights and Forecast - by Application
- 7.3.1. Neurovascular Diseases
- 7.3.2. Cardiovascular diseases
- 7.3.3. Others
- 7.1. Market Analysis, Insights and Forecast - by Product Technology Type
- 8. Asia Pacific Embolic Protection Systems Market Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Product Technology Type
- 8.1.1. Distal Occlusion Devices
- 8.1.2. Distal Filters
- 8.1.3. Proximal Occlusion Devices
- 8.2. Market Analysis, Insights and Forecast - by Material
- 8.2.1. Nitinol
- 8.2.2. Polyurethane
- 8.3. Market Analysis, Insights and Forecast - by Application
- 8.3.1. Neurovascular Diseases
- 8.3.2. Cardiovascular diseases
- 8.3.3. Others
- 8.1. Market Analysis, Insights and Forecast - by Product Technology Type
- 9. Middle East and Africa Embolic Protection Systems Market Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Product Technology Type
- 9.1.1. Distal Occlusion Devices
- 9.1.2. Distal Filters
- 9.1.3. Proximal Occlusion Devices
- 9.2. Market Analysis, Insights and Forecast - by Material
- 9.2.1. Nitinol
- 9.2.2. Polyurethane
- 9.3. Market Analysis, Insights and Forecast - by Application
- 9.3.1. Neurovascular Diseases
- 9.3.2. Cardiovascular diseases
- 9.3.3. Others
- 9.1. Market Analysis, Insights and Forecast - by Product Technology Type
- 10. South America Embolic Protection Systems Market Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Product Technology Type
- 10.1.1. Distal Occlusion Devices
- 10.1.2. Distal Filters
- 10.1.3. Proximal Occlusion Devices
- 10.2. Market Analysis, Insights and Forecast - by Material
- 10.2.1. Nitinol
- 10.2.2. Polyurethane
- 10.3. Market Analysis, Insights and Forecast - by Application
- 10.3.1. Neurovascular Diseases
- 10.3.2. Cardiovascular diseases
- 10.3.3. Others
- 10.1. Market Analysis, Insights and Forecast - by Product Technology Type
- 11. North America Embolic Protection Systems Market Analysis, Insights and Forecast, 2019-2031
- 11.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 11.1.1 United States
- 11.1.2 Canada
- 11.1.3 Mexico
- 12. Europe Embolic Protection Systems Market Analysis, Insights and Forecast, 2019-2031
- 12.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 12.1.1 Germany
- 12.1.2 United Kingdom
- 12.1.3 France
- 12.1.4 Italy
- 12.1.5 Spain
- 12.1.6 Rest of Europe
- 13. Asia Pacific Embolic Protection Systems Market Analysis, Insights and Forecast, 2019-2031
- 13.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 13.1.1 China
- 13.1.2 Japan
- 13.1.3 India
- 13.1.4 Australia
- 13.1.5 South Korea
- 13.1.6 Rest of Asia Pacific
- 14. Middle East and Africa Embolic Protection Systems Market Analysis, Insights and Forecast, 2019-2031
- 14.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 14.1.1 GCC
- 14.1.2 South Africa
- 14.1.3 Rest of Middle East and Africa
- 15. South America Embolic Protection Systems Market Analysis, Insights and Forecast, 2019-2031
- 15.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 15.1.1 Brazil
- 15.1.2 Argentina
- 15.1.3 Rest of South America
- 16. Competitive Analysis
- 16.1. Global Market Share Analysis 2024
- 16.2. Company Profiles
- 16.2.1 Edwards Lifesciences Corporation
- 16.2.1.1. Overview
- 16.2.1.2. Products
- 16.2.1.3. SWOT Analysis
- 16.2.1.4. Recent Developments
- 16.2.1.5. Financials (Based on Availability)
- 16.2.2 Silk Road MedicalInc
- 16.2.2.1. Overview
- 16.2.2.2. Products
- 16.2.2.3. SWOT Analysis
- 16.2.2.4. Recent Developments
- 16.2.2.5. Financials (Based on Availability)
- 16.2.3 Abbott Laboratories
- 16.2.3.1. Overview
- 16.2.3.2. Products
- 16.2.3.3. SWOT Analysis
- 16.2.3.4. Recent Developments
- 16.2.3.5. Financials (Based on Availability)
- 16.2.4 Contego Medical LLC
- 16.2.4.1. Overview
- 16.2.4.2. Products
- 16.2.4.3. SWOT Analysis
- 16.2.4.4. Recent Developments
- 16.2.4.5. Financials (Based on Availability)
- 16.2.5 Lepu Medical Technology*List Not Exhaustive
- 16.2.5.1. Overview
- 16.2.5.2. Products
- 16.2.5.3. SWOT Analysis
- 16.2.5.4. Recent Developments
- 16.2.5.5. Financials (Based on Availability)
- 16.2.6 Cardinal Health
- 16.2.6.1. Overview
- 16.2.6.2. Products
- 16.2.6.3. SWOT Analysis
- 16.2.6.4. Recent Developments
- 16.2.6.5. Financials (Based on Availability)
- 16.2.7 Hellman & Friedman LLC (Cordis)
- 16.2.7.1. Overview
- 16.2.7.2. Products
- 16.2.7.3. SWOT Analysis
- 16.2.7.4. Recent Developments
- 16.2.7.5. Financials (Based on Availability)
- 16.2.8 Medtronic Plc
- 16.2.8.1. Overview
- 16.2.8.2. Products
- 16.2.8.3. SWOT Analysis
- 16.2.8.4. Recent Developments
- 16.2.8.5. Financials (Based on Availability)
- 16.2.9 Boston Scientific Corporation
- 16.2.9.1. Overview
- 16.2.9.2. Products
- 16.2.9.3. SWOT Analysis
- 16.2.9.4. Recent Developments
- 16.2.9.5. Financials (Based on Availability)
- 16.2.1 Edwards Lifesciences Corporation
List of Figures
- Figure 1: Global Embolic Protection Systems Market Revenue Breakdown (Million, %) by Region 2024 & 2032
- Figure 2: North America Embolic Protection Systems Market Revenue (Million), by Country 2024 & 2032
- Figure 3: North America Embolic Protection Systems Market Revenue Share (%), by Country 2024 & 2032
- Figure 4: Europe Embolic Protection Systems Market Revenue (Million), by Country 2024 & 2032
- Figure 5: Europe Embolic Protection Systems Market Revenue Share (%), by Country 2024 & 2032
- Figure 6: Asia Pacific Embolic Protection Systems Market Revenue (Million), by Country 2024 & 2032
- Figure 7: Asia Pacific Embolic Protection Systems Market Revenue Share (%), by Country 2024 & 2032
- Figure 8: Middle East and Africa Embolic Protection Systems Market Revenue (Million), by Country 2024 & 2032
- Figure 9: Middle East and Africa Embolic Protection Systems Market Revenue Share (%), by Country 2024 & 2032
- Figure 10: South America Embolic Protection Systems Market Revenue (Million), by Country 2024 & 2032
- Figure 11: South America Embolic Protection Systems Market Revenue Share (%), by Country 2024 & 2032
- Figure 12: North America Embolic Protection Systems Market Revenue (Million), by Product Technology Type 2024 & 2032
- Figure 13: North America Embolic Protection Systems Market Revenue Share (%), by Product Technology Type 2024 & 2032
- Figure 14: North America Embolic Protection Systems Market Revenue (Million), by Material 2024 & 2032
- Figure 15: North America Embolic Protection Systems Market Revenue Share (%), by Material 2024 & 2032
- Figure 16: North America Embolic Protection Systems Market Revenue (Million), by Application 2024 & 2032
- Figure 17: North America Embolic Protection Systems Market Revenue Share (%), by Application 2024 & 2032
- Figure 18: North America Embolic Protection Systems Market Revenue (Million), by Country 2024 & 2032
- Figure 19: North America Embolic Protection Systems Market Revenue Share (%), by Country 2024 & 2032
- Figure 20: Europe Embolic Protection Systems Market Revenue (Million), by Product Technology Type 2024 & 2032
- Figure 21: Europe Embolic Protection Systems Market Revenue Share (%), by Product Technology Type 2024 & 2032
- Figure 22: Europe Embolic Protection Systems Market Revenue (Million), by Material 2024 & 2032
- Figure 23: Europe Embolic Protection Systems Market Revenue Share (%), by Material 2024 & 2032
- Figure 24: Europe Embolic Protection Systems Market Revenue (Million), by Application 2024 & 2032
- Figure 25: Europe Embolic Protection Systems Market Revenue Share (%), by Application 2024 & 2032
- Figure 26: Europe Embolic Protection Systems Market Revenue (Million), by Country 2024 & 2032
- Figure 27: Europe Embolic Protection Systems Market Revenue Share (%), by Country 2024 & 2032
- Figure 28: Asia Pacific Embolic Protection Systems Market Revenue (Million), by Product Technology Type 2024 & 2032
- Figure 29: Asia Pacific Embolic Protection Systems Market Revenue Share (%), by Product Technology Type 2024 & 2032
- Figure 30: Asia Pacific Embolic Protection Systems Market Revenue (Million), by Material 2024 & 2032
- Figure 31: Asia Pacific Embolic Protection Systems Market Revenue Share (%), by Material 2024 & 2032
- Figure 32: Asia Pacific Embolic Protection Systems Market Revenue (Million), by Application 2024 & 2032
- Figure 33: Asia Pacific Embolic Protection Systems Market Revenue Share (%), by Application 2024 & 2032
- Figure 34: Asia Pacific Embolic Protection Systems Market Revenue (Million), by Country 2024 & 2032
- Figure 35: Asia Pacific Embolic Protection Systems Market Revenue Share (%), by Country 2024 & 2032
- Figure 36: Middle East and Africa Embolic Protection Systems Market Revenue (Million), by Product Technology Type 2024 & 2032
- Figure 37: Middle East and Africa Embolic Protection Systems Market Revenue Share (%), by Product Technology Type 2024 & 2032
- Figure 38: Middle East and Africa Embolic Protection Systems Market Revenue (Million), by Material 2024 & 2032
- Figure 39: Middle East and Africa Embolic Protection Systems Market Revenue Share (%), by Material 2024 & 2032
- Figure 40: Middle East and Africa Embolic Protection Systems Market Revenue (Million), by Application 2024 & 2032
- Figure 41: Middle East and Africa Embolic Protection Systems Market Revenue Share (%), by Application 2024 & 2032
- Figure 42: Middle East and Africa Embolic Protection Systems Market Revenue (Million), by Country 2024 & 2032
- Figure 43: Middle East and Africa Embolic Protection Systems Market Revenue Share (%), by Country 2024 & 2032
- Figure 44: South America Embolic Protection Systems Market Revenue (Million), by Product Technology Type 2024 & 2032
- Figure 45: South America Embolic Protection Systems Market Revenue Share (%), by Product Technology Type 2024 & 2032
- Figure 46: South America Embolic Protection Systems Market Revenue (Million), by Material 2024 & 2032
- Figure 47: South America Embolic Protection Systems Market Revenue Share (%), by Material 2024 & 2032
- Figure 48: South America Embolic Protection Systems Market Revenue (Million), by Application 2024 & 2032
- Figure 49: South America Embolic Protection Systems Market Revenue Share (%), by Application 2024 & 2032
- Figure 50: South America Embolic Protection Systems Market Revenue (Million), by Country 2024 & 2032
- Figure 51: South America Embolic Protection Systems Market Revenue Share (%), by Country 2024 & 2032
List of Tables
- Table 1: Global Embolic Protection Systems Market Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Global Embolic Protection Systems Market Revenue Million Forecast, by Product Technology Type 2019 & 2032
- Table 3: Global Embolic Protection Systems Market Revenue Million Forecast, by Material 2019 & 2032
- Table 4: Global Embolic Protection Systems Market Revenue Million Forecast, by Application 2019 & 2032
- Table 5: Global Embolic Protection Systems Market Revenue Million Forecast, by Region 2019 & 2032
- Table 6: Global Embolic Protection Systems Market Revenue Million Forecast, by Country 2019 & 2032
- Table 7: United States Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 8: Canada Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 9: Mexico Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 10: Global Embolic Protection Systems Market Revenue Million Forecast, by Country 2019 & 2032
- Table 11: Germany Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 12: United Kingdom Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 13: France Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 14: Italy Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 15: Spain Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 16: Rest of Europe Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 17: Global Embolic Protection Systems Market Revenue Million Forecast, by Country 2019 & 2032
- Table 18: China Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 19: Japan Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 20: India Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 21: Australia Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 22: South Korea Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 23: Rest of Asia Pacific Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 24: Global Embolic Protection Systems Market Revenue Million Forecast, by Country 2019 & 2032
- Table 25: GCC Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 26: South Africa Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 27: Rest of Middle East and Africa Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 28: Global Embolic Protection Systems Market Revenue Million Forecast, by Country 2019 & 2032
- Table 29: Brazil Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 30: Argentina Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 31: Rest of South America Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 32: Global Embolic Protection Systems Market Revenue Million Forecast, by Product Technology Type 2019 & 2032
- Table 33: Global Embolic Protection Systems Market Revenue Million Forecast, by Material 2019 & 2032
- Table 34: Global Embolic Protection Systems Market Revenue Million Forecast, by Application 2019 & 2032
- Table 35: Global Embolic Protection Systems Market Revenue Million Forecast, by Country 2019 & 2032
- Table 36: United States Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 37: Canada Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 38: Mexico Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 39: Global Embolic Protection Systems Market Revenue Million Forecast, by Product Technology Type 2019 & 2032
- Table 40: Global Embolic Protection Systems Market Revenue Million Forecast, by Material 2019 & 2032
- Table 41: Global Embolic Protection Systems Market Revenue Million Forecast, by Application 2019 & 2032
- Table 42: Global Embolic Protection Systems Market Revenue Million Forecast, by Country 2019 & 2032
- Table 43: Germany Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 44: United Kingdom Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 45: France Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 46: Italy Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 47: Spain Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 48: Rest of Europe Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 49: Global Embolic Protection Systems Market Revenue Million Forecast, by Product Technology Type 2019 & 2032
- Table 50: Global Embolic Protection Systems Market Revenue Million Forecast, by Material 2019 & 2032
- Table 51: Global Embolic Protection Systems Market Revenue Million Forecast, by Application 2019 & 2032
- Table 52: Global Embolic Protection Systems Market Revenue Million Forecast, by Country 2019 & 2032
- Table 53: China Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 54: Japan Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 55: India Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 56: Australia Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 57: South Korea Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 58: Rest of Asia Pacific Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 59: Global Embolic Protection Systems Market Revenue Million Forecast, by Product Technology Type 2019 & 2032
- Table 60: Global Embolic Protection Systems Market Revenue Million Forecast, by Material 2019 & 2032
- Table 61: Global Embolic Protection Systems Market Revenue Million Forecast, by Application 2019 & 2032
- Table 62: Global Embolic Protection Systems Market Revenue Million Forecast, by Country 2019 & 2032
- Table 63: GCC Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 64: South Africa Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 65: Rest of Middle East and Africa Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 66: Global Embolic Protection Systems Market Revenue Million Forecast, by Product Technology Type 2019 & 2032
- Table 67: Global Embolic Protection Systems Market Revenue Million Forecast, by Material 2019 & 2032
- Table 68: Global Embolic Protection Systems Market Revenue Million Forecast, by Application 2019 & 2032
- Table 69: Global Embolic Protection Systems Market Revenue Million Forecast, by Country 2019 & 2032
- Table 70: Brazil Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 71: Argentina Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 72: Rest of South America Embolic Protection Systems Market Revenue (Million) Forecast, by Application 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Embolic Protection Systems Market?
The projected CAGR is approximately 8.60%.
2. Which companies are prominent players in the Embolic Protection Systems Market?
Key companies in the market include Edwards Lifesciences Corporation, Silk Road MedicalInc, Abbott Laboratories, Contego Medical LLC, Lepu Medical Technology*List Not Exhaustive, Cardinal Health, Hellman & Friedman LLC (Cordis), Medtronic Plc, Boston Scientific Corporation.
3. What are the main segments of the Embolic Protection Systems Market?
The market segments include Product Technology Type, Material, Application.
4. Can you provide details about the market size?
The market size is estimated to be USD XX Million as of 2022.
5. What are some drivers contributing to market growth?
Rise in Prevalence of Cardiovascular and Neurovascular Diseases; Increasing Demand for Minimally Invasive Surgeries.
6. What are the notable trends driving market growth?
Cardiovascular Disease Segment is Expected to Witness a Notable Growth Over the Forecast period.
7. Are there any restraints impacting market growth?
Stringent Regulatory Scenarios and Unfavorable Government Policies; Lack of Skilled Labor.
8. Can you provide examples of recent developments in the market?
In November 2022, Innovative Cardiovascular Solutions sponsored a clinical study to evaluate the safety, effectiveness, and performance of the EMBLOK embolic protection systems (EPS) during transcatheter aortic valve replacement (TAVR) by randomized comparison with a commercially available embolic protection device.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Embolic Protection Systems Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Embolic Protection Systems Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Embolic Protection Systems Market?
To stay informed about further developments, trends, and reports in the Embolic Protection Systems Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


