Key Insights
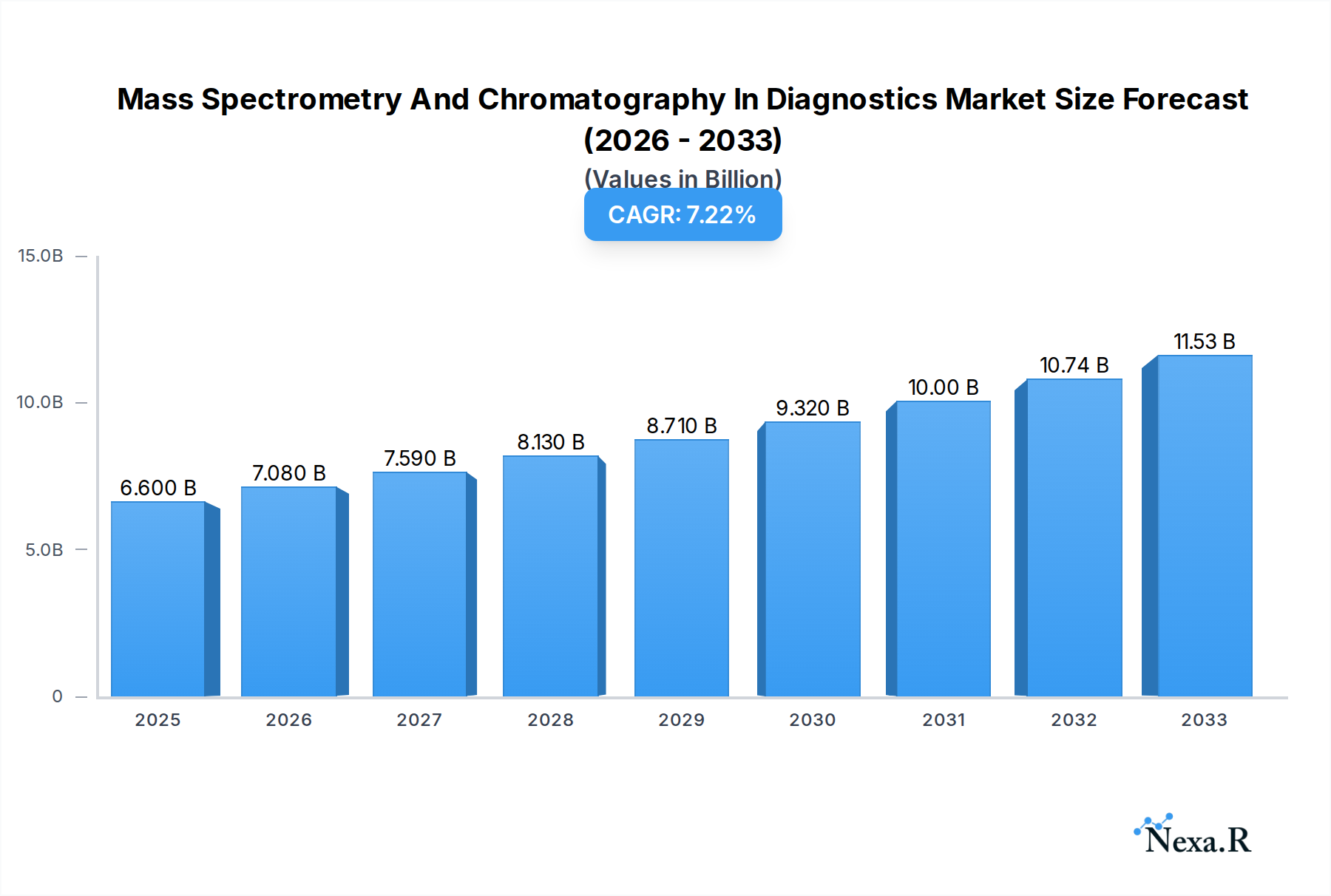
The global Mass Spectrometry and Chromatography in Diagnostics market is poised for substantial growth, with an estimated market size of $6.6 billion in 2025. This expansion is driven by an anticipated Compound Annual Growth Rate (CAGR) of 7.2% during the forecast period of 2025-2033. A significant factor propelling this market is the increasing demand for precise and sensitive diagnostic tools across various healthcare applications, including commercial testing and advanced laboratory analyses. The inherent capabilities of mass spectrometry and chromatography in identifying and quantifying biomarkers with high accuracy are becoming indispensable for early disease detection, personalized medicine, and therapeutic drug monitoring. Furthermore, ongoing technological advancements, leading to more portable, user-friendly, and cost-effective instruments, are broadening their accessibility and adoption in both established and emerging markets. The rising global healthcare expenditure and the growing prevalence of chronic and infectious diseases are also contributing to this robust market trajectory.
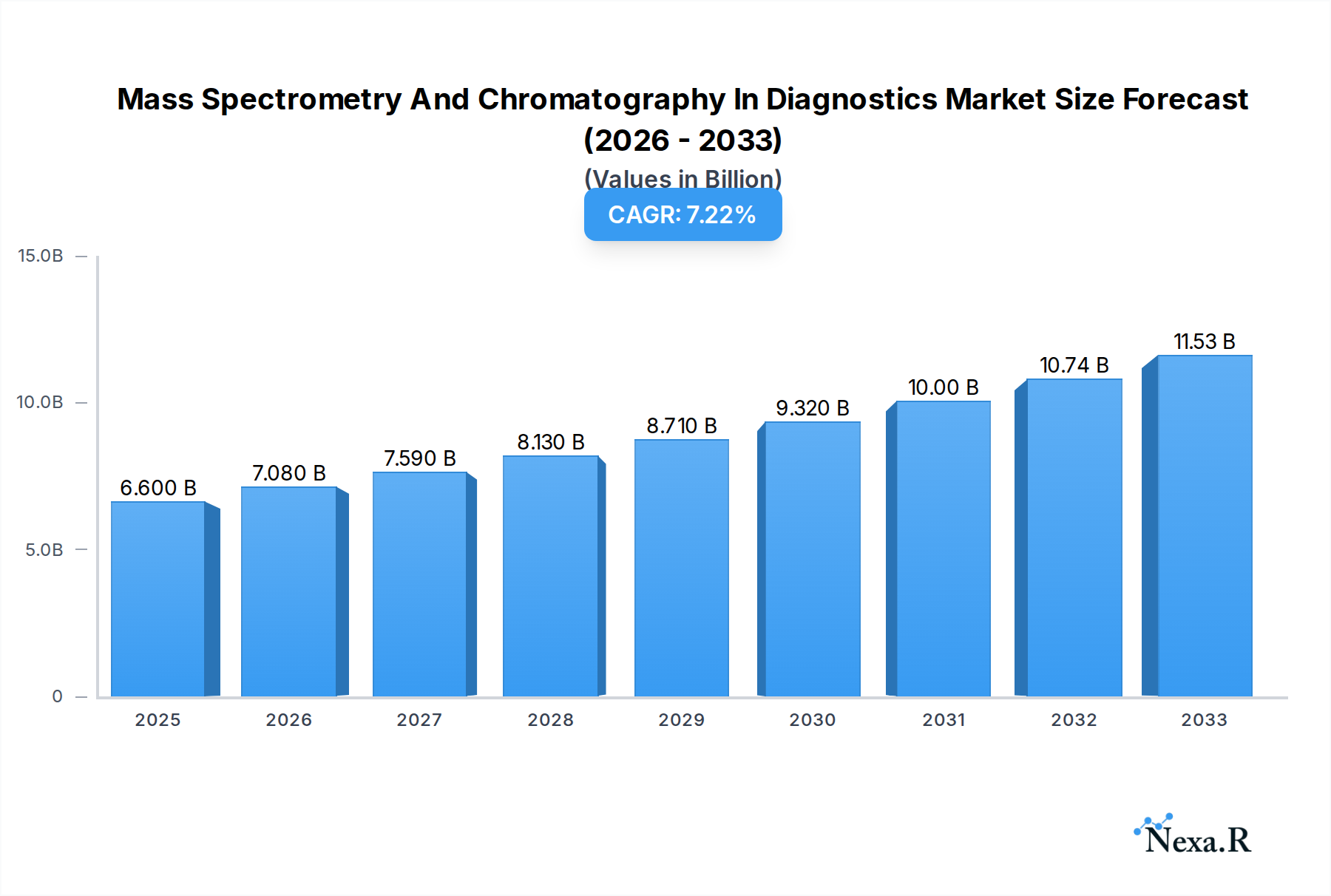
Mass Spectrometry And Chromatography In Diagnostics Market Size (In Billion)

The market is segmented into distinct application areas, with Commercial Testing and Laboratories representing key growth segments. Within these, Mass Spectrometry and Chromatography are the dominant technological types, each offering unique analytical advantages. Key players such as Agilent Technologies, Thermo Fisher Scientific, and Waters Corporation are at the forefront of innovation, consistently introducing advanced solutions. While the market exhibits strong growth potential, certain factors could influence its pace. These include the high initial investment cost for sophisticated equipment, the need for skilled personnel to operate and maintain these systems, and stringent regulatory approval processes for new diagnostic assays. Despite these challenges, the overarching trend towards precision diagnostics, coupled with the relentless pursuit of enhanced analytical performance, solidifies a positive outlook for the Mass Spectrometry and Chromatography in Diagnostics market. The Asia Pacific region, with its rapidly developing healthcare infrastructure and increasing R&D investments, is expected to emerge as a significant growth hub.
Mass Spectrometry And Chromatography In Diagnostics Company Market Share

Mass Spectrometry And Chromatography In Diagnostics: Comprehensive Market Analysis 2019–2033
This in-depth report provides a thorough examination of the global Mass Spectrometry and Chromatography in Diagnostics market, encompassing historical trends, current dynamics, and future projections. With the global diagnostics market projected to reach $XXX billion by 2033, driven by advancements in clinical diagnostics, biotechnology, and pharmaceutical research, this report offers critical insights for stakeholders. We analyze the intricate interplay of mass spectrometry applications and chromatography techniques within diverse diagnostic settings, including commercial testing and laboratory diagnostics. Key industry players such as Agilent Technologies, Thermo Fisher Scientific, and Waters Corporation are scrutinized for their strategic contributions and market influence. This report is essential for understanding market concentration, technological innovation drivers, regulatory frameworks, and emerging opportunities, crucial for strategic decision-making in the rapidly evolving in vitro diagnostics (IVD) landscape.
Mass Spectrometry And Chromatography In Diagnostics Market Dynamics & Structure
The Mass Spectrometry and Chromatography in Diagnostics market exhibits a moderately consolidated structure, with a significant market share held by key players including Agilent Technologies, Thermo Fisher Scientific, and Waters Corporation. Technological innovation acts as a primary driver, with continuous advancements in high-resolution mass spectrometry (HRMS) and ultra-high-performance liquid chromatography (UHPLC) enhancing sensitivity, specificity, and throughput in diagnostic assays. Regulatory frameworks, particularly those set by the FDA and EMA, play a pivotal role in market access and product development, emphasizing safety and efficacy. Competitive product substitutes, while present, often lack the comprehensive analytical power of mass spectrometry and chromatography for complex biomarker identification and quantification. End-user demographics are increasingly sophisticated, with a growing demand from research institutions, clinical laboratories, and contract research organizations (CROs) seeking advanced analytical solutions for diseases such as cancer, infectious diseases, and neurological disorders. Mergers and acquisition (M&A) trends indicate strategic consolidation, with larger entities acquiring innovative startups to expand their portfolios and market reach. For instance, Danaher Corporation’s strategic acquisitions have consistently bolstered its presence in the life sciences and diagnostics sectors.
- Market Concentration: Moderately consolidated with a few dominant players.
- Technological Innovation Drivers: Advancements in HRMS, UHPLC, and integrated LC-MS/MS systems.
- Regulatory Frameworks: Strict adherence to FDA, EMA, and other regional regulatory bodies for IVD approval.
- Competitive Product Substitutes: Molecular diagnostics and immunoassay-based tests offer alternatives for specific applications but lack the depth of MS and chromatography.
- End-User Demographics: Growing demand from academic research, clinical diagnostics, pharmaceutical R&D, and forensic science.
- M&A Trends: Strategic acquisitions to gain access to novel technologies and expand market share.
Mass Spectrometry And Chromatography In Diagnostics Growth Trends & Insights
The global Mass Spectrometry and Chromatography in Diagnostics market is poised for robust growth, projected to expand at a significant Compound Annual Growth Rate (CAGR) of approximately XX% from 2025 to 2033. This upward trajectory is underpinned by a substantial increase in the market size, expected to grow from an estimated $XX billion in 2025 to $XXX billion by 2033. The historical period (2019–2024) witnessed consistent expansion, driven by increasing awareness of personalized medicine and the growing need for precise diagnostic tools. Adoption rates for advanced analytical techniques are accelerating, particularly in the commercial testing and laboratory segments, as healthcare providers and researchers recognize their ability to detect and quantify biomarkers with unparalleled accuracy. Technological disruptions, such as the miniaturization of mass spectrometers and the development of portable chromatography systems, are further democratizing access to these powerful analytical instruments, enabling their use in point-of-care settings. Consumer behavior shifts are also contributing, with a growing demand for comprehensive health screenings and early disease detection. This evolution is propelling the market towards more sophisticated proteomics, metabolomics, and genomics analyses for a wide range of diagnostic applications.
The base year of 2025 stands as a crucial benchmark, with preliminary estimates placing the market value at $XX billion. The estimated year also being 2025, allows for a direct comparison to the starting point of the forecast period. The forecast period (2025–2033) is anticipated to be characterized by sustained growth, fueled by ongoing research and development in novel diagnostic assays and the expanding applications of mass spectrometry and chromatography in identifying complex disease signatures. For instance, the application of LC-MS/MS in therapeutic drug monitoring (TDM) and the identification of biomarkers for neurodegenerative diseases are key growth areas. Furthermore, the integration of AI and machine learning with mass spectrometry data analysis is revolutionizing the interpretation of complex datasets, leading to faster and more accurate diagnoses. This technological synergy is expected to significantly drive market penetration across various sub-segments of the in vitro diagnostics (IVD) industry. The historical growth from 2019 to 2024, though not precisely quantified here, laid the foundation for this projected expansion by establishing the utility and value of these advanced analytical techniques in clinical settings.
Dominant Regions, Countries, or Segments in Mass Spectrometry And Chromatography In Diagnostics
North America currently stands as the dominant region in the Mass Spectrometry and Chromatography in Diagnostics market, driven by a confluence of factors including a robust healthcare infrastructure, substantial investments in R&D, and the early adoption of advanced diagnostic technologies. The United States, in particular, commands a significant market share due to a high prevalence of chronic diseases, a well-established network of research institutions and biopharmaceutical companies, and favorable government funding for scientific research. The commercial testing segment within North America is a major contributor, fueled by a growing demand for comprehensive health assessments and early disease detection services.
Within the Type segmentation, both Mass Spectrometry and Chromatography are critical, with Mass Spectrometry applications experiencing particularly rapid growth due to its unparalleled sensitivity and specificity in biomarker discovery and validation. The integration of LC-MS/MS systems for clinical applications, such as in neonatal screening, toxicology, and infectious disease diagnostics, further solidifies its dominance. Shimadzu Corporation and Tecan Group are among the key players contributing to the strength of the North American market through their innovative product offerings and strategic partnerships.
- Dominant Region: North America.
- Dominant Country: United States.
- Key Application Segment Driving Growth: Commercial Testing.
- Key Type Segment Driving Growth: Mass Spectrometry.
Factors contributing to North America's dominance include:
- Economic Policies: Significant government and private sector investment in healthcare and life sciences research.
- Infrastructure: Advanced laboratory facilities and a high density of healthcare professionals trained in utilizing sophisticated analytical techniques.
- Technological Advancement: Early adoption and development of cutting-edge mass spectrometry and chromatography technologies.
- Disease Burden: High prevalence of chronic and lifestyle-related diseases necessitating advanced diagnostic solutions.
- Regulatory Environment: A mature regulatory framework that supports innovation while ensuring patient safety.
The Laboratory segment also plays a crucial role, with academic and clinical laboratories increasingly adopting these advanced techniques for a wide array of diagnostic purposes, from routine testing to complex research-driven investigations. The growth potential in other regions, such as Europe and Asia Pacific, is also significant, driven by expanding healthcare access and increasing investments in R&D, suggesting a future shift in market dynamics. However, for the forecast period, North America is expected to maintain its leadership position due to its established ecosystem and continuous innovation.
Mass Spectrometry And Chromatography In Diagnostics Product Landscape
The product landscape for Mass Spectrometry and Chromatography in Diagnostics is characterized by continuous innovation aimed at enhancing analytical performance, user-friendliness, and cost-effectiveness. Leading companies like Agilent Technologies, Thermo Fisher Scientific, and Waters Corporation are at the forefront, offering a diverse range of instruments including high-resolution mass spectrometers, advanced gas and liquid chromatography systems, and integrated LC-MS/MS platforms. These products are designed to address critical needs in clinical diagnostics, drug discovery, and food safety, offering superior sensitivity, specificity, and throughput for biomarker identification and quantification. Unique selling propositions often lie in proprietary ionization techniques, advanced detector technologies, and sophisticated software solutions that facilitate complex data analysis. Technological advancements are focused on miniaturization, automation, and the development of benchtop and portable systems, expanding accessibility and application in diverse settings.
Key Drivers, Barriers & Challenges in Mass Spectrometry And Chromatography In Diagnostics
Key Drivers
The Mass Spectrometry and Chromatography in Diagnostics market is propelled by several key drivers. Technological advancements in areas like high-resolution mass spectrometry and UHPLC enable unprecedented accuracy in biomarker detection, crucial for early disease diagnosis. Increasing prevalence of chronic diseases such as cancer, diabetes, and neurological disorders fuels the demand for more precise and comprehensive diagnostic tools. Growing investments in pharmaceutical R&D and personalized medicine necessitate advanced analytical capabilities for drug development and patient stratification. Furthermore, supportive government initiatives and funding for healthcare and scientific research accelerate innovation and market adoption.
Barriers & Challenges
Despite the growth, the market faces significant barriers and challenges. High initial cost and complexity of instrumentation can be a deterrent for smaller laboratories. Stringent regulatory approval processes for diagnostic assays and instruments can delay market entry and increase development costs. Shortage of skilled personnel trained to operate and interpret data from these advanced systems poses a considerable challenge. Supply chain disruptions, as witnessed in recent global events, can impact the availability of critical components. Limited standardization in data formats and analytical methods across different platforms can also hinder widespread adoption and inter-laboratory comparability.
Emerging Opportunities in Mass Spectrometry And Chromatography In Diagnostics
Emerging opportunities in the Mass Spectrometry and Chromatography in Diagnostics market are largely driven by the burgeoning fields of personalized medicine and advanced biomarker discovery. The expansion of liquid biopsy techniques for cancer detection and monitoring presents a significant avenue, as these methods rely heavily on the sensitivity of mass spectrometry and chromatography to detect low-abundance biomarkers in bodily fluids. Another key opportunity lies in the growing application of these technologies in infectious disease diagnostics, particularly in identifying novel pathogens and antibiotic resistance markers. The development of point-of-care diagnostics incorporating miniaturized MS and chromatography systems also holds immense potential for rapid, on-site testing. Furthermore, the increasing integration of artificial intelligence (AI) and machine learning with MS and chromatography data analysis is unlocking new possibilities for predictive diagnostics and a deeper understanding of disease mechanisms.
Growth Accelerators in the Mass Spectrometry And Chromatography In Diagnostics Industry
Several factors are acting as significant growth accelerators for the Mass Spectrometry and Chromatography in Diagnostics industry. Technological breakthroughs in areas such as ion mobility spectrometry-mass spectrometry (IMS-MS) and advanced chromatography consumables are continuously enhancing analytical capabilities, leading to improved diagnostic accuracy and efficiency. Strategic partnerships and collaborations between instrument manufacturers, diagnostic test developers, and academic research institutions are fostering innovation and accelerating the translation of research findings into clinical applications. The increasing focus on preventative healthcare and early disease detection worldwide is creating a robust demand for advanced diagnostic solutions, directly benefiting the MS and chromatography market. Moreover, expanding applications in non-clinical areas such as environmental monitoring and food safety analysis are further diversifying revenue streams and driving market expansion. The proactive efforts of companies like Merck and Bio-Rad Laboratories in developing integrated solutions and expanding their global reach are also contributing to this accelerated growth.
Key Players Shaping the Mass Spectrometry And Chromatography In Diagnostics Market
- Agilent Technologies
- Thermo Fisher Scientific
- Waters Corporation
- Tecan Group
- Danaher Corporation
- Shimadzu Corporation
- Merck
- Bio-Rad Laboratories
- Promega Corporation
- Restek Corporation
- Phenomenex
- Zivak
Notable Milestones in Mass Spectrometry And Chromatography In Diagnostics Sector
- 2020: Thermo Fisher Scientific launches a new Orbitrap Exploris 480 mass spectrometer, enhancing high-resolution capabilities for routine clinical research.
- 2021: Agilent Technologies acquires a leading provider of chromatography consumables, expanding its portfolio and market reach.
- 2022: Waters Corporation introduces a next-generation mass spectrometer designed for enhanced protein quantification in diagnostic applications.
- 2022: Tecan Group announces a strategic partnership to develop automated solutions for MS-based diagnostic testing.
- 2023: Danaher Corporation's subsidiary, Cytiva, acquires a company specializing in advanced bioprocessing solutions, indirectly benefiting the diagnostics sector.
- 2023: Shimadzu Corporation unveils an integrated LC-MS system optimized for high-throughput clinical laboratory workflows.
- 2024: Merck introduces novel reagents and kits designed to improve the efficiency of sample preparation for MS-based diagnostics.
In-Depth Mass Spectrometry And Chromatography In Diagnostics Market Outlook
The outlook for the Mass Spectrometry and Chromatography in Diagnostics market remains exceptionally positive, with sustained growth anticipated throughout the forecast period. Key growth accelerators, including continuous technological innovation and the expanding applications in personalized medicine and early disease detection, will continue to fuel market expansion. Strategic initiatives by key players, such as Promega Corporation and Restek Corporation, in developing novel consumables and advanced analytical platforms, will further enhance the capabilities and accessibility of these technologies. The increasing emphasis on preventative healthcare globally, coupled with significant investments in R&D by leading nations and corporations, presents substantial opportunities for market penetration and revenue growth. The convergence of mass spectrometry and chromatography with artificial intelligence is poised to revolutionize diagnostic capabilities, leading to more accurate and timely disease identification and management, thereby solidifying the indispensable role of these analytical techniques in the future of healthcare.
Mass Spectrometry And Chromatography In Diagnostics Segmentation
-
1. Application
- 1.1. Commercial Testing
- 1.2. Laboratory
- 1.3. Others
-
2. Type
- 2.1. Mass Spectrometry
- 2.2. Chromatography
Mass Spectrometry And Chromatography In Diagnostics Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. South America
- 2.1. Brazil
- 2.2. Argentina
- 2.3. Rest of South America
-
3. Europe
- 3.1. United Kingdom
- 3.2. Germany
- 3.3. France
- 3.4. Italy
- 3.5. Spain
- 3.6. Russia
- 3.7. Benelux
- 3.8. Nordics
- 3.9. Rest of Europe
-
4. Middle East & Africa
- 4.1. Turkey
- 4.2. Israel
- 4.3. GCC
- 4.4. North Africa
- 4.5. South Africa
- 4.6. Rest of Middle East & Africa
-
5. Asia Pacific
- 5.1. China
- 5.2. India
- 5.3. Japan
- 5.4. South Korea
- 5.5. ASEAN
- 5.6. Oceania
- 5.7. Rest of Asia Pacific
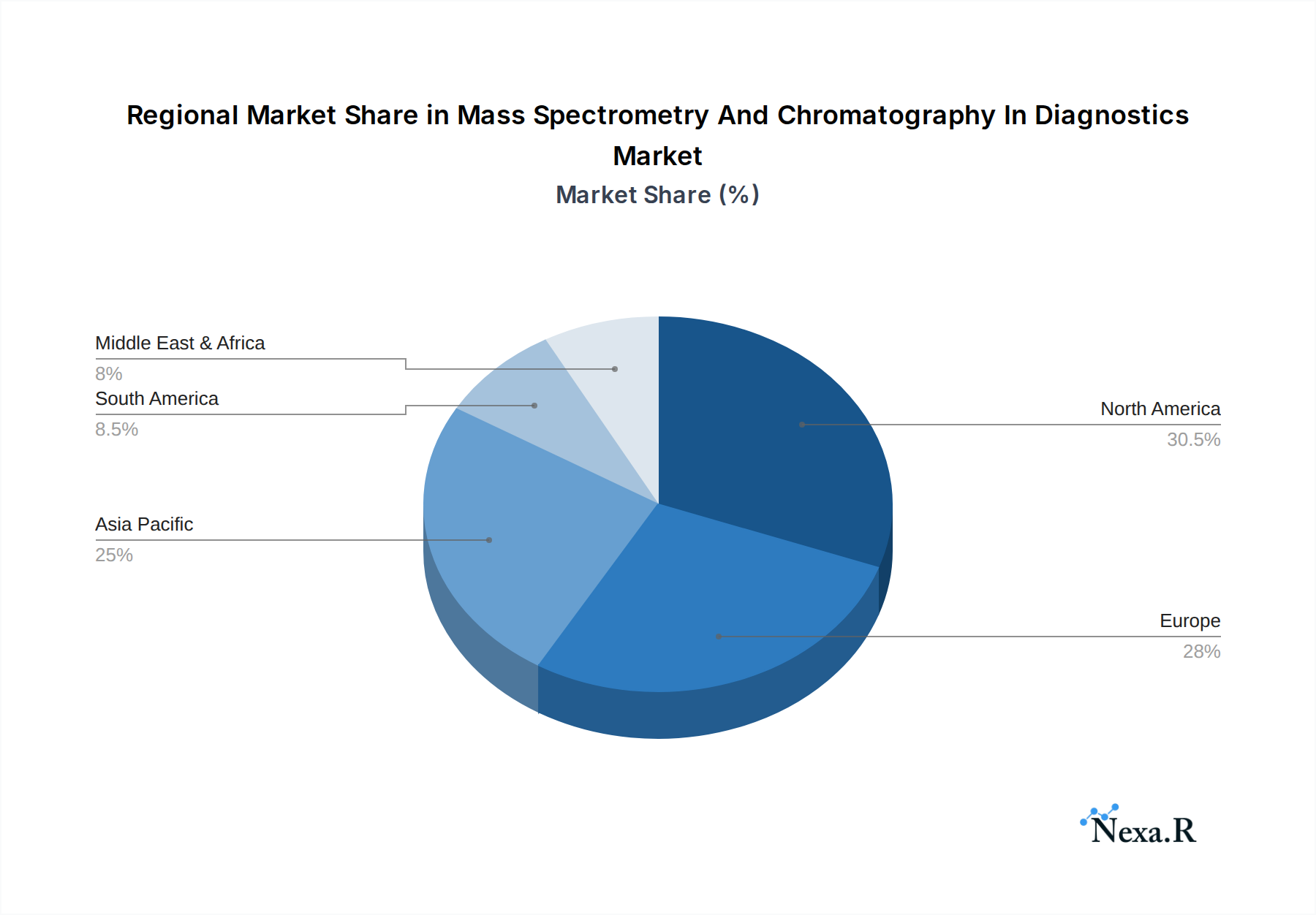
Mass Spectrometry And Chromatography In Diagnostics Regional Market Share

Geographic Coverage of Mass Spectrometry And Chromatography In Diagnostics
Mass Spectrometry And Chromatography In Diagnostics REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 7.2% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.3. Market Restrains
- 3.4. Market Trends
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Mass Spectrometry And Chromatography In Diagnostics Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Application
- 5.1.1. Commercial Testing
- 5.1.2. Laboratory
- 5.1.3. Others
- 5.2. Market Analysis, Insights and Forecast - by Type
- 5.2.1. Mass Spectrometry
- 5.2.2. Chromatography
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. South America
- 5.3.3. Europe
- 5.3.4. Middle East & Africa
- 5.3.5. Asia Pacific
- 5.1. Market Analysis, Insights and Forecast - by Application
- 6. North America Mass Spectrometry And Chromatography In Diagnostics Analysis, Insights and Forecast, 2020-2032
- 6.1. Market Analysis, Insights and Forecast - by Application
- 6.1.1. Commercial Testing
- 6.1.2. Laboratory
- 6.1.3. Others
- 6.2. Market Analysis, Insights and Forecast - by Type
- 6.2.1. Mass Spectrometry
- 6.2.2. Chromatography
- 6.1. Market Analysis, Insights and Forecast - by Application
- 7. South America Mass Spectrometry And Chromatography In Diagnostics Analysis, Insights and Forecast, 2020-2032
- 7.1. Market Analysis, Insights and Forecast - by Application
- 7.1.1. Commercial Testing
- 7.1.2. Laboratory
- 7.1.3. Others
- 7.2. Market Analysis, Insights and Forecast - by Type
- 7.2.1. Mass Spectrometry
- 7.2.2. Chromatography
- 7.1. Market Analysis, Insights and Forecast - by Application
- 8. Europe Mass Spectrometry And Chromatography In Diagnostics Analysis, Insights and Forecast, 2020-2032
- 8.1. Market Analysis, Insights and Forecast - by Application
- 8.1.1. Commercial Testing
- 8.1.2. Laboratory
- 8.1.3. Others
- 8.2. Market Analysis, Insights and Forecast - by Type
- 8.2.1. Mass Spectrometry
- 8.2.2. Chromatography
- 8.1. Market Analysis, Insights and Forecast - by Application
- 9. Middle East & Africa Mass Spectrometry And Chromatography In Diagnostics Analysis, Insights and Forecast, 2020-2032
- 9.1. Market Analysis, Insights and Forecast - by Application
- 9.1.1. Commercial Testing
- 9.1.2. Laboratory
- 9.1.3. Others
- 9.2. Market Analysis, Insights and Forecast - by Type
- 9.2.1. Mass Spectrometry
- 9.2.2. Chromatography
- 9.1. Market Analysis, Insights and Forecast - by Application
- 10. Asia Pacific Mass Spectrometry And Chromatography In Diagnostics Analysis, Insights and Forecast, 2020-2032
- 10.1. Market Analysis, Insights and Forecast - by Application
- 10.1.1. Commercial Testing
- 10.1.2. Laboratory
- 10.1.3. Others
- 10.2. Market Analysis, Insights and Forecast - by Type
- 10.2.1. Mass Spectrometry
- 10.2.2. Chromatography
- 10.1. Market Analysis, Insights and Forecast - by Application
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2025
- 11.2. Company Profiles
- 11.2.1 Agilent Technologies
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Thermo Fisher Scientific
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 Waters Corporation
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Tecan Group
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Danaher Corporation
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Shimadzu Corporation
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 Merck
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Bio-Rad Laboratories
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 Promega Corporation
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Restek Corporation
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 Phenomenex
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.12 Zivak
- 11.2.12.1. Overview
- 11.2.12.2. Products
- 11.2.12.3. SWOT Analysis
- 11.2.12.4. Recent Developments
- 11.2.12.5. Financials (Based on Availability)
- 11.2.1 Agilent Technologies
List of Figures
- Figure 1: Global Mass Spectrometry And Chromatography In Diagnostics Revenue Breakdown (undefined, %) by Region 2025 & 2033
- Figure 2: North America Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined), by Application 2025 & 2033
- Figure 3: North America Mass Spectrometry And Chromatography In Diagnostics Revenue Share (%), by Application 2025 & 2033
- Figure 4: North America Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined), by Type 2025 & 2033
- Figure 5: North America Mass Spectrometry And Chromatography In Diagnostics Revenue Share (%), by Type 2025 & 2033
- Figure 6: North America Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined), by Country 2025 & 2033
- Figure 7: North America Mass Spectrometry And Chromatography In Diagnostics Revenue Share (%), by Country 2025 & 2033
- Figure 8: South America Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined), by Application 2025 & 2033
- Figure 9: South America Mass Spectrometry And Chromatography In Diagnostics Revenue Share (%), by Application 2025 & 2033
- Figure 10: South America Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined), by Type 2025 & 2033
- Figure 11: South America Mass Spectrometry And Chromatography In Diagnostics Revenue Share (%), by Type 2025 & 2033
- Figure 12: South America Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined), by Country 2025 & 2033
- Figure 13: South America Mass Spectrometry And Chromatography In Diagnostics Revenue Share (%), by Country 2025 & 2033
- Figure 14: Europe Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined), by Application 2025 & 2033
- Figure 15: Europe Mass Spectrometry And Chromatography In Diagnostics Revenue Share (%), by Application 2025 & 2033
- Figure 16: Europe Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined), by Type 2025 & 2033
- Figure 17: Europe Mass Spectrometry And Chromatography In Diagnostics Revenue Share (%), by Type 2025 & 2033
- Figure 18: Europe Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined), by Country 2025 & 2033
- Figure 19: Europe Mass Spectrometry And Chromatography In Diagnostics Revenue Share (%), by Country 2025 & 2033
- Figure 20: Middle East & Africa Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined), by Application 2025 & 2033
- Figure 21: Middle East & Africa Mass Spectrometry And Chromatography In Diagnostics Revenue Share (%), by Application 2025 & 2033
- Figure 22: Middle East & Africa Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined), by Type 2025 & 2033
- Figure 23: Middle East & Africa Mass Spectrometry And Chromatography In Diagnostics Revenue Share (%), by Type 2025 & 2033
- Figure 24: Middle East & Africa Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined), by Country 2025 & 2033
- Figure 25: Middle East & Africa Mass Spectrometry And Chromatography In Diagnostics Revenue Share (%), by Country 2025 & 2033
- Figure 26: Asia Pacific Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined), by Application 2025 & 2033
- Figure 27: Asia Pacific Mass Spectrometry And Chromatography In Diagnostics Revenue Share (%), by Application 2025 & 2033
- Figure 28: Asia Pacific Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined), by Type 2025 & 2033
- Figure 29: Asia Pacific Mass Spectrometry And Chromatography In Diagnostics Revenue Share (%), by Type 2025 & 2033
- Figure 30: Asia Pacific Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined), by Country 2025 & 2033
- Figure 31: Asia Pacific Mass Spectrometry And Chromatography In Diagnostics Revenue Share (%), by Country 2025 & 2033
List of Tables
- Table 1: Global Mass Spectrometry And Chromatography In Diagnostics Revenue undefined Forecast, by Application 2020 & 2033
- Table 2: Global Mass Spectrometry And Chromatography In Diagnostics Revenue undefined Forecast, by Type 2020 & 2033
- Table 3: Global Mass Spectrometry And Chromatography In Diagnostics Revenue undefined Forecast, by Region 2020 & 2033
- Table 4: Global Mass Spectrometry And Chromatography In Diagnostics Revenue undefined Forecast, by Application 2020 & 2033
- Table 5: Global Mass Spectrometry And Chromatography In Diagnostics Revenue undefined Forecast, by Type 2020 & 2033
- Table 6: Global Mass Spectrometry And Chromatography In Diagnostics Revenue undefined Forecast, by Country 2020 & 2033
- Table 7: United States Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 8: Canada Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 9: Mexico Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 10: Global Mass Spectrometry And Chromatography In Diagnostics Revenue undefined Forecast, by Application 2020 & 2033
- Table 11: Global Mass Spectrometry And Chromatography In Diagnostics Revenue undefined Forecast, by Type 2020 & 2033
- Table 12: Global Mass Spectrometry And Chromatography In Diagnostics Revenue undefined Forecast, by Country 2020 & 2033
- Table 13: Brazil Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 14: Argentina Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 15: Rest of South America Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 16: Global Mass Spectrometry And Chromatography In Diagnostics Revenue undefined Forecast, by Application 2020 & 2033
- Table 17: Global Mass Spectrometry And Chromatography In Diagnostics Revenue undefined Forecast, by Type 2020 & 2033
- Table 18: Global Mass Spectrometry And Chromatography In Diagnostics Revenue undefined Forecast, by Country 2020 & 2033
- Table 19: United Kingdom Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 20: Germany Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 21: France Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 22: Italy Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 23: Spain Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 24: Russia Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 25: Benelux Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 26: Nordics Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 27: Rest of Europe Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 28: Global Mass Spectrometry And Chromatography In Diagnostics Revenue undefined Forecast, by Application 2020 & 2033
- Table 29: Global Mass Spectrometry And Chromatography In Diagnostics Revenue undefined Forecast, by Type 2020 & 2033
- Table 30: Global Mass Spectrometry And Chromatography In Diagnostics Revenue undefined Forecast, by Country 2020 & 2033
- Table 31: Turkey Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 32: Israel Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 33: GCC Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 34: North Africa Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 35: South Africa Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 36: Rest of Middle East & Africa Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 37: Global Mass Spectrometry And Chromatography In Diagnostics Revenue undefined Forecast, by Application 2020 & 2033
- Table 38: Global Mass Spectrometry And Chromatography In Diagnostics Revenue undefined Forecast, by Type 2020 & 2033
- Table 39: Global Mass Spectrometry And Chromatography In Diagnostics Revenue undefined Forecast, by Country 2020 & 2033
- Table 40: China Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 41: India Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 42: Japan Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 43: South Korea Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 44: ASEAN Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 45: Oceania Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 46: Rest of Asia Pacific Mass Spectrometry And Chromatography In Diagnostics Revenue (undefined) Forecast, by Application 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Mass Spectrometry And Chromatography In Diagnostics?
The projected CAGR is approximately 7.2%.
2. Which companies are prominent players in the Mass Spectrometry And Chromatography In Diagnostics?
Key companies in the market include Agilent Technologies, Thermo Fisher Scientific, Waters Corporation, Tecan Group, Danaher Corporation, Shimadzu Corporation, Merck, Bio-Rad Laboratories, Promega Corporation, Restek Corporation, Phenomenex, Zivak.
3. What are the main segments of the Mass Spectrometry And Chromatography In Diagnostics?
The market segments include Application, Type.
4. Can you provide details about the market size?
The market size is estimated to be USD XXX N/A as of 2022.
5. What are some drivers contributing to market growth?
N/A
6. What are the notable trends driving market growth?
N/A
7. Are there any restraints impacting market growth?
N/A
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3950.00, USD 5925.00, and USD 7900.00 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in N/A.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Mass Spectrometry And Chromatography In Diagnostics," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Mass Spectrometry And Chromatography In Diagnostics report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Mass Spectrometry And Chromatography In Diagnostics?
To stay informed about further developments, trends, and reports in the Mass Spectrometry And Chromatography In Diagnostics, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database
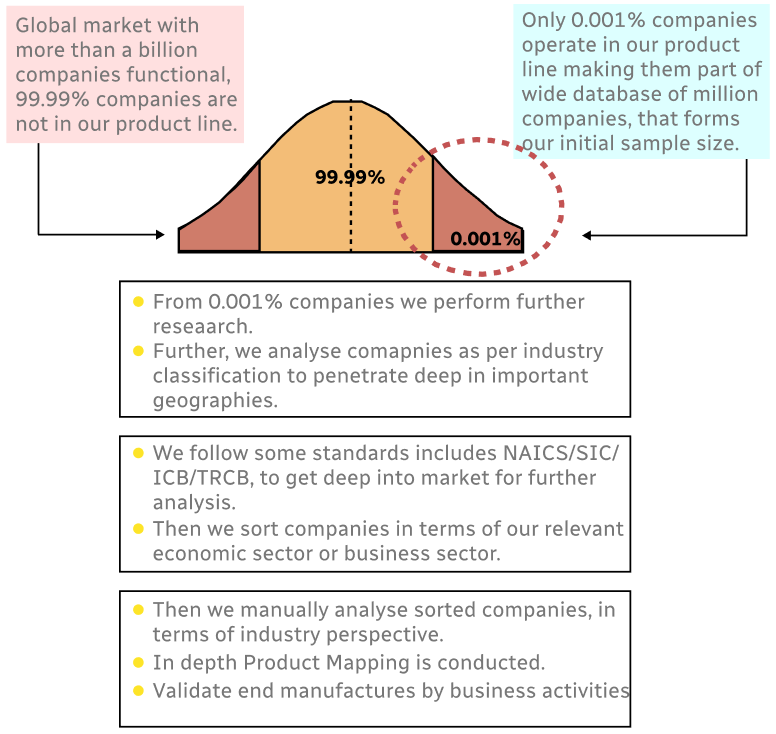
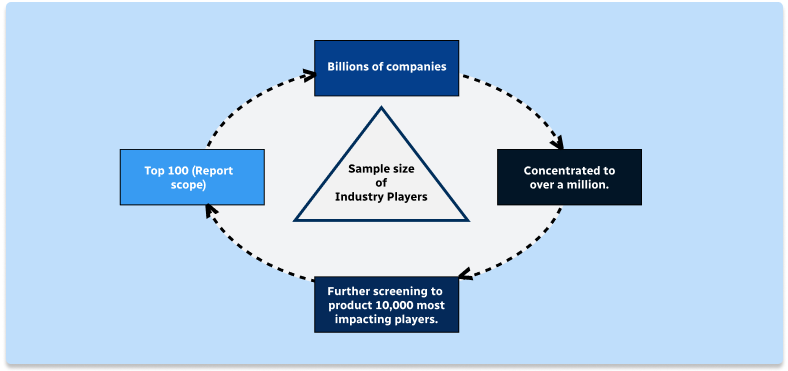
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
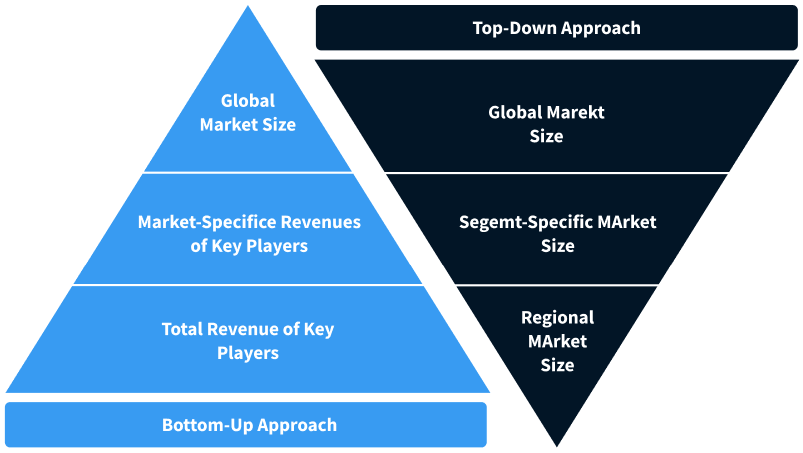
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
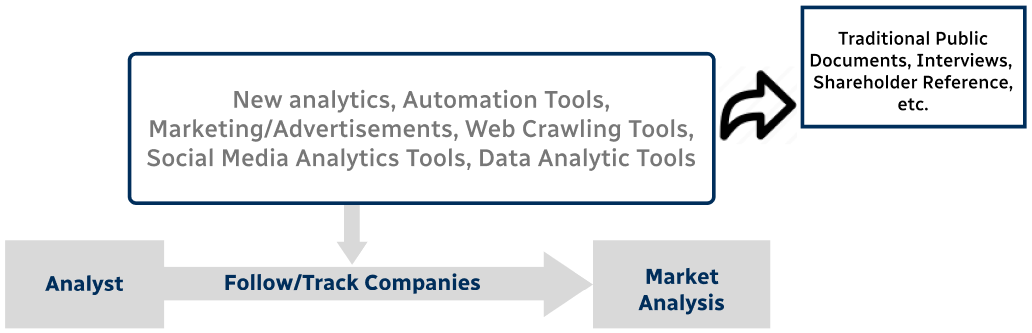
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


