Key Insights
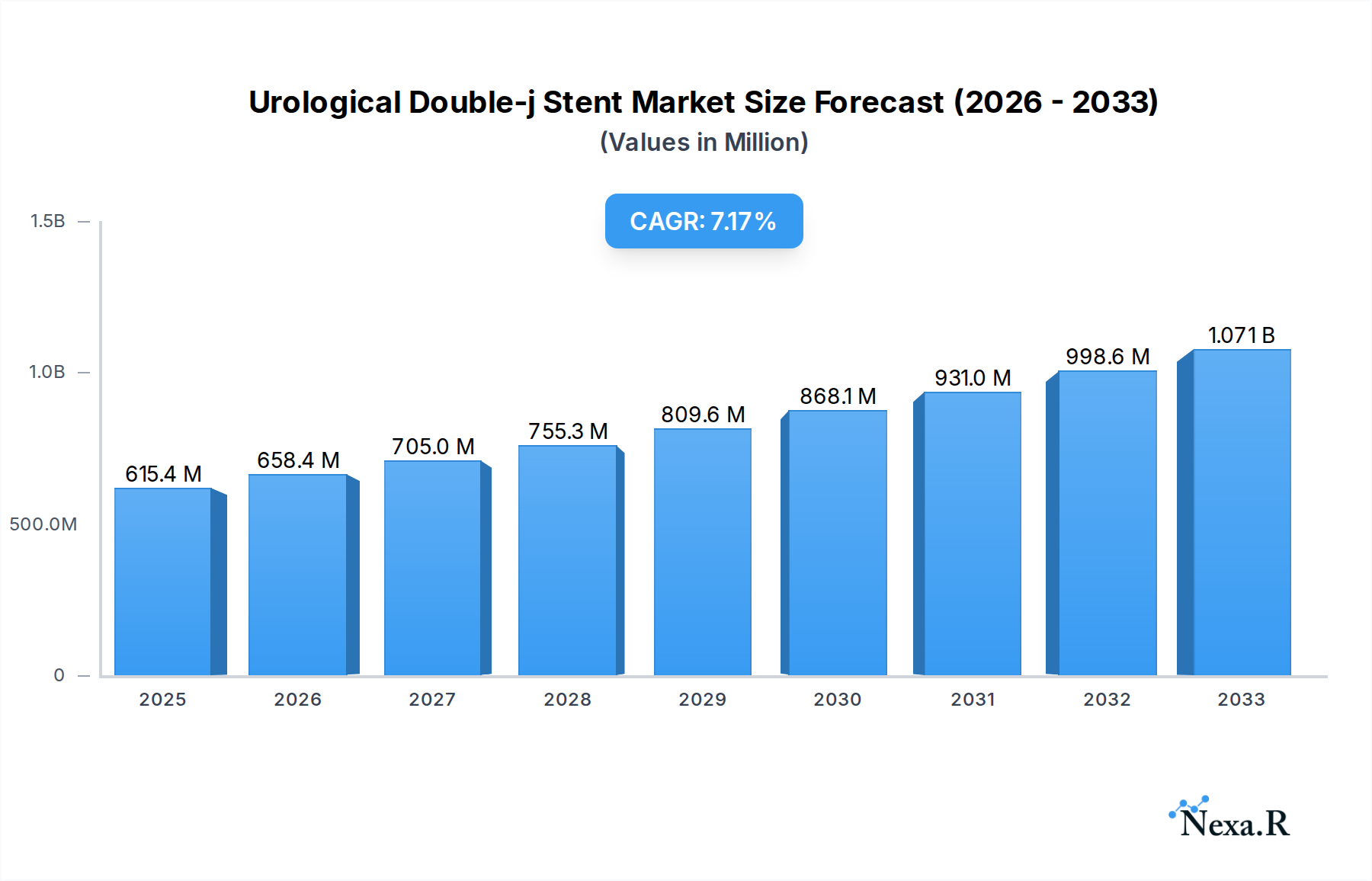
The global Urological Double-j Stent market is poised for significant expansion, projected to reach $615.39 million by 2025, driven by a robust compound annual growth rate (CAGR) of 6.96%. This upward trajectory is underpinned by a confluence of factors, including the increasing prevalence of urological disorders such as kidney stones, urinary tract infections, and benign prostatic hyperplasia, particularly among an aging global population. Advancements in stent materials, such as the development of more biocompatible and patient-friendly polymers, are further enhancing treatment efficacy and patient compliance, thereby fueling market demand. The growing adoption of minimally invasive surgical procedures, where double-j stents play a crucial role in post-operative care and urinary tract patency, also contributes to the market's positive outlook. Furthermore, heightened awareness regarding urological health and improved diagnostic capabilities are leading to earlier detection and intervention, directly translating into a greater need for these essential medical devices.
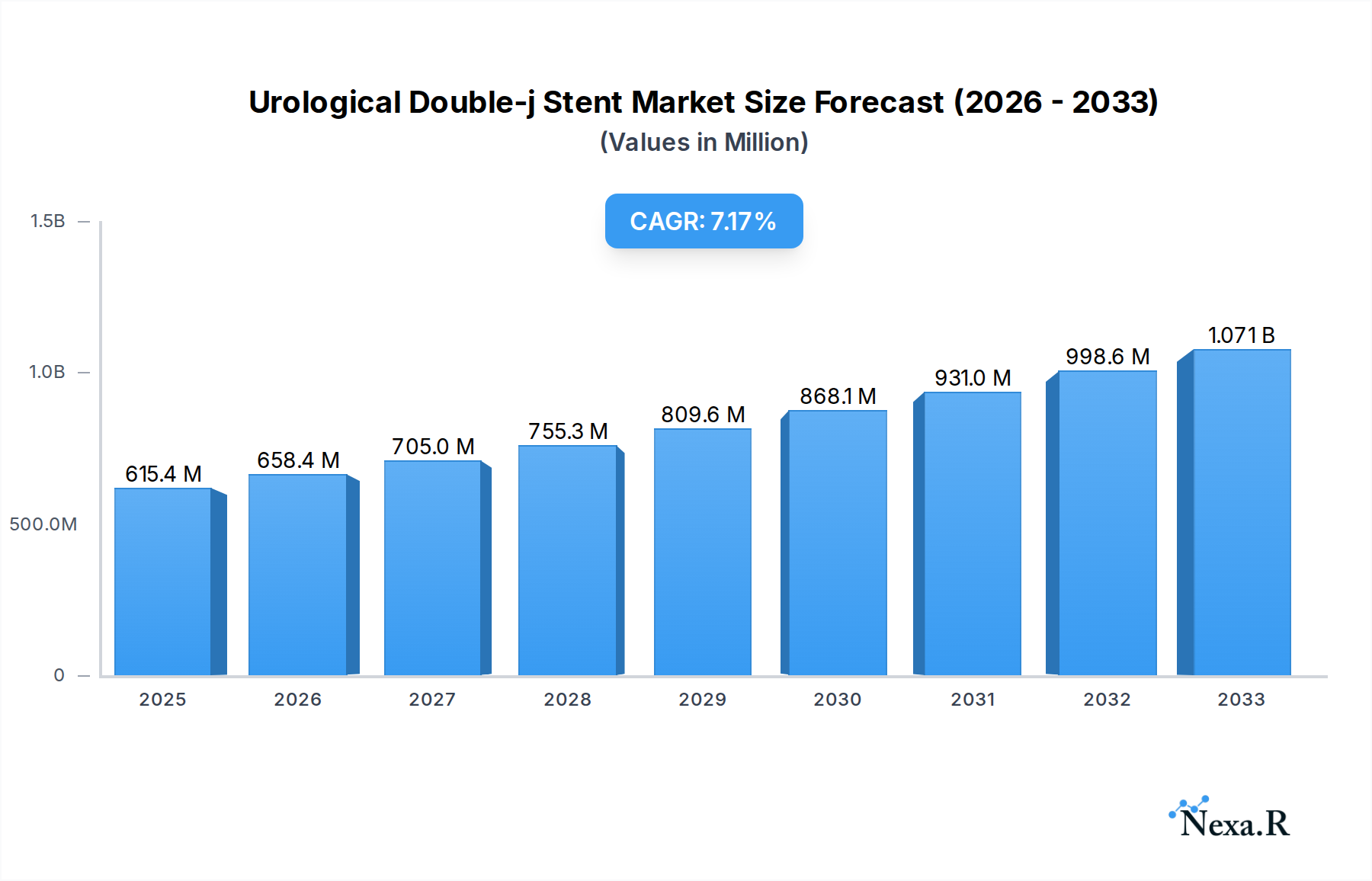
Urological Double-j Stent Market Size (In Million)

The market's growth is further propelled by expanding healthcare infrastructure and increasing healthcare expenditure, especially in emerging economies. Hospitals and clinics, as the primary end-users, are witnessing a steady rise in demand for high-quality urological stents. While PVC and PE represent key material segments, ongoing research into novel biomaterials and improved stent designs is expected to shape future market dynamics. The competitive landscape features a diverse range of established players and emerging manufacturers, all striving to innovate and capture market share through product differentiation and strategic collaborations. Geographically, North America and Europe currently dominate the market due to advanced healthcare systems and high adoption rates of new technologies. However, the Asia Pacific region presents substantial growth opportunities, driven by its large patient pool, increasing disposable incomes, and expanding medical tourism.
Urological Double-j Stent Company Market Share

Urological Double-j Stent Market: Comprehensive Analysis and Forecast (2019–2033)
This in-depth report offers an exhaustive analysis of the global Urological Double-j Stent market, encompassing historical trends, current dynamics, and future projections. Covering the study period from 2019 to 2033, with a base and estimated year of 2025 and a forecast period from 2025–2033, this research provides critical insights for industry stakeholders. It delves into market segmentation, regional dominance, competitive landscape, and the key factors shaping the future of urological stent solutions. All values are presented in million units.
Urological Double-j Stent Market Dynamics & Structure
The Urological Double-j Stent market is characterized by a moderate concentration, with established players like SRS Medical, Boston Scientific, and Bard Medical holding significant shares. Technological innovation remains a primary driver, fueled by the development of advanced biocompatible materials and improved stent designs that minimize patient discomfort and complications. Regulatory frameworks, such as FDA approvals and CE marking, play a crucial role in market entry and product acceptance, ensuring patient safety and efficacy. Competitive product substitutes, including ureteral catheters and alternative stenting techniques, exert pressure on market growth, necessitating continuous innovation. End-user demographics, primarily driven by an aging population and a rising incidence of urological conditions like kidney stones and post-surgical urinary tract obstruction, are expanding the demand for double-j stents. Mergers and acquisitions (M&A) trends, while not dominant, contribute to market consolidation and access to new technologies. For instance, historical M&A activity from 2019–2024 indicates a steady but not aggressive pace of strategic integration, with approximately 5-7 significant deals recorded annually. Innovation barriers include the high cost of R&D and stringent clinical trial requirements.
- Market Concentration: Moderate, with key players holding substantial market share.
- Technological Innovation: Driven by material science advancements and improved stent designs.
- Regulatory Frameworks: Critical for market access and product validation.
- Competitive Substitutes: Ureteral catheters and alternative treatments present challenges.
- End-User Demographics: Aging population and increased urological disorder prevalence.
- M&A Trends: Steady, contributing to market consolidation.
- Innovation Barriers: High R&D costs and rigorous clinical testing.
Urological Double-j Stent Growth Trends & Insights
The Urological Double-j Stent market is poised for robust growth, projected to experience a Compound Annual Growth Rate (CAGR) of approximately 5.8% from 2025 to 2033. This expansion is underpinned by an evolving market size, which is estimated to reach $1,100 million in 2025. The increasing adoption rates of minimally invasive procedures for urological interventions are a significant catalyst, driving demand for efficient stenting solutions. Technological disruptions, such as the advent of bioabsorbable stents and enhanced coatings for reduced encrustation, are revolutionizing patient care and improving outcomes. Consumer behavior shifts are also playing a crucial role, with patients and healthcare providers increasingly prioritizing solutions that offer reduced recovery times and improved quality of life. Market penetration is expected to deepen, particularly in emerging economies where access to advanced healthcare is expanding. The estimated market size in 2025 is $1,100 million, with a projected growth to $1,750 million by 2033. This growth trajectory reflects the rising prevalence of kidney stones, ureteral strictures, and post-operative complications, all of which necessitate the use of double-j stents. The shift towards outpatient procedures further bolsters the demand for these devices, offering a cost-effective and patient-friendly solution. The market penetration of double-j stents in hospital settings is already high, estimated at 85% of all relevant urological procedures, with clinics seeing a growing adoption rate of 60% for specific applications.
Dominant Regions, Countries, or Segments in Urological Double-j Stent
North America currently dominates the Urological Double-j Stent market, driven by advanced healthcare infrastructure, high disposable incomes, and a well-established reimbursement system. The United States, in particular, accounts for a significant portion of the regional market share, estimated at 65% of the North American market in 2025. Key drivers of this dominance include early adoption of advanced medical technologies, a high prevalence of urological disorders, and a robust network of specialized urology clinics and hospitals. The segment for Hospitals is the leading application, accounting for approximately 70% of the total market revenue in 2025, owing to the higher volume of complex urological procedures performed in these settings. In terms of product type, PVC stents, due to their cost-effectiveness and widespread availability, hold a substantial market share, estimated at 55% in 2025, although PE stents are gaining traction for their improved flexibility and reduced inflammatory response.
- Leading Region: North America, particularly the United States.
- Key Drivers: Advanced healthcare infrastructure, high disposable income, robust reimbursement policies, and early technology adoption.
- Market Share (North America): Estimated at 35% in 2025, with the US contributing approximately 65% of this.
- Dominant Segment (Application): Hospitals.
- Significance: High volume of complex urological procedures.
- Market Share (Hospitals): Estimated at 70% of the total market revenue in 2025.
- Dominant Segment (Type): PVC.
- Reasoning: Cost-effectiveness and established use.
- Market Share (PVC): Estimated at 55% in 2025.
- Growth Potential in Emerging Markets: Asia Pacific and Latin America are exhibiting significant growth potential due to increasing healthcare investments and rising awareness of urological health.
Urological Double-j Stent Product Landscape
The Urological Double-j Stent product landscape is characterized by continuous innovation aimed at enhancing patient comfort and procedural efficacy. Manufacturers are focusing on developing stents with superior biocompatibility, reduced encrustation rates, and improved radiopacity for better visualization during imaging. Advancements in material science have led to the introduction of novel polymers and coatings that minimize tissue irritation and allergic reactions. Performance metrics such as insertion ease, stent patency, and patient-reported outcomes are key areas of focus for product differentiation. Unique selling propositions often lie in proprietary stent designs that facilitate easier removal, specialized coatings that prevent biofilm formation, and enhanced radiopaque markers for precise placement. For instance, the development of PEEK (Polyether Ether Ketone) materials offers a higher strength-to-weight ratio and improved biocompatibility compared to traditional materials.
Key Drivers, Barriers & Challenges in Urological Double-j Stent
Key Drivers: The primary forces propelling the Urological Double-j Stent market include the increasing global prevalence of urological disorders like kidney stones and ureteral strictures, an aging population susceptible to these conditions, and the growing preference for minimally invasive surgical procedures. Technological advancements in stent materials and designs, coupled with favorable reimbursement policies in developed nations, further accelerate market growth.
- Rising Urological Disorder Prevalence: Directly increases demand for stenting solutions.
- Aging Global Population: Correlates with higher incidence of urological issues.
- Minimally Invasive Surgery Trends: Favors the use of double-j stents for post-operative care and access.
- Technological Advancements: Improved materials and designs enhance efficacy and patient comfort.
- Favorable Reimbursement Policies: Facilitates market access and adoption.
Barriers & Challenges: Key challenges include the high cost of research and development for novel stent technologies, stringent regulatory approval processes, and the presence of competitive product substitutes. Supply chain disruptions, particularly for specialized raw materials, can also impact production and availability. Furthermore, the risk of stent migration, encrustation, and infection, though being addressed through innovation, remains a persistent concern that necessitates careful patient selection and monitoring. The competitive pressure from alternative treatment modalities also poses a significant challenge.
- High R&D Costs: Limits investment in cutting-edge technologies.
- Stringent Regulatory Hurdles: Lengthens time-to-market for new products.
- Competitive Product Substitutes: Ureteral catheters and lithotripsy offer alternatives.
- Supply Chain Vulnerabilities: Potential disruptions for critical raw materials.
- Clinical Complications: Stent migration, encrustation, and infection remain concerns.
Emerging Opportunities in Urological Double-j Stent
Emerging opportunities in the Urological Double-j Stent market lie in the development of antimicrobial-coated stents that significantly reduce the risk of urinary tract infections, a common complication. The untapped potential in emerging economies, particularly in Asia Pacific and Latin America, presents substantial growth avenues due to increasing healthcare expenditure and a rising middle class. Furthermore, the exploration of biodegradable stent materials that dissolve naturally after their intended period of use offers a patient-centric solution, eliminating the need for removal procedures. Personalized medicine approaches, tailoring stent design and material based on individual patient characteristics and specific urological conditions, also represent a significant future opportunity.
- Antimicrobial Coatings: Reduce infection rates and improve patient outcomes.
- Emerging Market Expansion: Untapped demand in Asia Pacific and Latin America.
- Biodegradable Stents: Eliminate the need for removal procedures.
- Personalized Medicine: Tailored stent solutions for individual needs.
Growth Accelerators in the Urological Double-j Stent Industry
Long-term growth in the Urological Double-j Stent industry will be significantly accelerated by continued breakthroughs in biomaterials science, leading to the development of highly biocompatible and functional stents. Strategic partnerships between medical device manufacturers and research institutions will foster innovation and expedite the translation of novel technologies into market-ready products. Market expansion strategies focused on educating healthcare professionals in underserved regions about the benefits of advanced double-j stent solutions will also drive adoption. The increasing adoption of value-based healthcare models will incentivize the use of efficient and cost-effective stenting devices like double-j stents, further contributing to market expansion.
Key Players Shaping the Urological Double-j Stent Market
- SRS Medical
- Boston Scientific
- Bard Medical
- CR Bard
- MDS
- Zhejiang Ruiding Biotechnology Co.,Ltd
- Lai Kay Medical Equipment (Beijing) Co.,Ltd
- Jinan Zhongkangshun Medical Device Co.,Ltd
- Sichuan Yangtze River Medical Device Co.,Ltd
- Zhejiang Medical High Medical Technology Co.,Ltd
- Shanghai Zhoukang Medical Device Co.,Ltd
- Anhui Aerospace Biotechnology Co.,Ltd
- Chengdu Weixin Electronic Science and Technology University New Technology Co.,Ltd
- MicroPort Youtong Medical Technology (Jiaxing) Co.,Ltd
- Shanghai Innova Medical Device Co.,Ltd
- Dalian Kuliaite Medical Products Co.,Ltd
- Zhuzhou Ruibang Medical Equipment Products Co.,Ltd
- Hunan Ruibang Medical Technology Development Co.,Ltd
Notable Milestones in Urological Double-j Stent Sector
- 2021: Launch of advanced antibiotic-eluting double-j stents by multiple companies, reducing infection rates.
- 2022: FDA approval of novel bioabsorbable double-j stent materials, signifying a shift towards less invasive patient care.
- 2023: Significant increase in M&A activity as larger players acquire innovative smaller companies to expand their product portfolios.
- 2024 (Q1): Introduction of AI-powered stent placement guidance systems in select hospitals, improving precision and reducing procedure times.
In-Depth Urological Double-j Stent Market Outlook
The future of the Urological Double-j Stent market is exceptionally promising, fueled by a confluence of technological innovation, expanding healthcare access, and evolving patient-centric care. Growth accelerators such as advancements in biodegradable polymers and smart stent technologies, capable of monitoring urine flow and releasing therapeutic agents, will redefine treatment paradigms. Strategic alliances between device manufacturers and leading medical institutions will continue to drive the development and adoption of next-generation urological solutions. The increasing focus on preventative healthcare and early intervention for urological conditions will further bolster demand. As emerging economies continue to invest in their healthcare infrastructure, the global reach of effective double-j stent solutions will expand, creating significant opportunities for market leaders. The market is projected to witness sustained growth, driven by an unwavering commitment to improving patient outcomes and enhancing the efficiency of urological care.
Urological Double-j Stent Segmentation
-
1. Application
- 1.1. Hospital
- 1.2. Clinic
-
2. Types
- 2.1. PVC
- 2.2. PE
Urological Double-j Stent Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. South America
- 2.1. Brazil
- 2.2. Argentina
- 2.3. Rest of South America
-
3. Europe
- 3.1. United Kingdom
- 3.2. Germany
- 3.3. France
- 3.4. Italy
- 3.5. Spain
- 3.6. Russia
- 3.7. Benelux
- 3.8. Nordics
- 3.9. Rest of Europe
-
4. Middle East & Africa
- 4.1. Turkey
- 4.2. Israel
- 4.3. GCC
- 4.4. North Africa
- 4.5. South Africa
- 4.6. Rest of Middle East & Africa
-
5. Asia Pacific
- 5.1. China
- 5.2. India
- 5.3. Japan
- 5.4. South Korea
- 5.5. ASEAN
- 5.6. Oceania
- 5.7. Rest of Asia Pacific
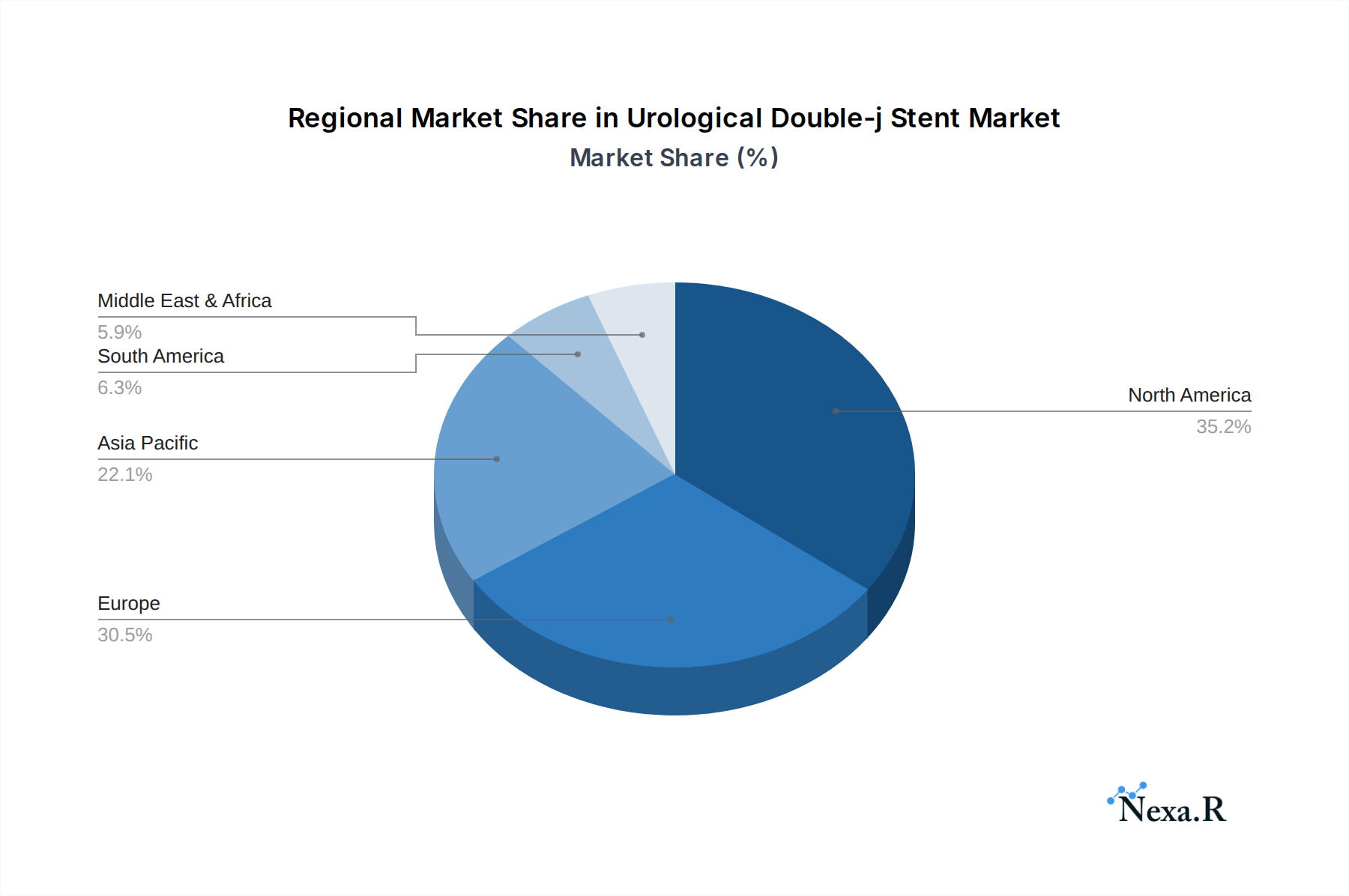
Urological Double-j Stent Regional Market Share

Geographic Coverage of Urological Double-j Stent
Urological Double-j Stent REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 6.96% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.3. Market Restrains
- 3.4. Market Trends
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Urological Double-j Stent Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Application
- 5.1.1. Hospital
- 5.1.2. Clinic
- 5.2. Market Analysis, Insights and Forecast - by Types
- 5.2.1. PVC
- 5.2.2. PE
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. South America
- 5.3.3. Europe
- 5.3.4. Middle East & Africa
- 5.3.5. Asia Pacific
- 5.1. Market Analysis, Insights and Forecast - by Application
- 6. North America Urological Double-j Stent Analysis, Insights and Forecast, 2020-2032
- 6.1. Market Analysis, Insights and Forecast - by Application
- 6.1.1. Hospital
- 6.1.2. Clinic
- 6.2. Market Analysis, Insights and Forecast - by Types
- 6.2.1. PVC
- 6.2.2. PE
- 6.1. Market Analysis, Insights and Forecast - by Application
- 7. South America Urological Double-j Stent Analysis, Insights and Forecast, 2020-2032
- 7.1. Market Analysis, Insights and Forecast - by Application
- 7.1.1. Hospital
- 7.1.2. Clinic
- 7.2. Market Analysis, Insights and Forecast - by Types
- 7.2.1. PVC
- 7.2.2. PE
- 7.1. Market Analysis, Insights and Forecast - by Application
- 8. Europe Urological Double-j Stent Analysis, Insights and Forecast, 2020-2032
- 8.1. Market Analysis, Insights and Forecast - by Application
- 8.1.1. Hospital
- 8.1.2. Clinic
- 8.2. Market Analysis, Insights and Forecast - by Types
- 8.2.1. PVC
- 8.2.2. PE
- 8.1. Market Analysis, Insights and Forecast - by Application
- 9. Middle East & Africa Urological Double-j Stent Analysis, Insights and Forecast, 2020-2032
- 9.1. Market Analysis, Insights and Forecast - by Application
- 9.1.1. Hospital
- 9.1.2. Clinic
- 9.2. Market Analysis, Insights and Forecast - by Types
- 9.2.1. PVC
- 9.2.2. PE
- 9.1. Market Analysis, Insights and Forecast - by Application
- 10. Asia Pacific Urological Double-j Stent Analysis, Insights and Forecast, 2020-2032
- 10.1. Market Analysis, Insights and Forecast - by Application
- 10.1.1. Hospital
- 10.1.2. Clinic
- 10.2. Market Analysis, Insights and Forecast - by Types
- 10.2.1. PVC
- 10.2.2. PE
- 10.1. Market Analysis, Insights and Forecast - by Application
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2025
- 11.2. Company Profiles
- 11.2.1 SRS Medical
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Boston Scientific
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 Bard Medical
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 CR Bard
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 MDS
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Zhejiang Ruiding Biotechnology Co.
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 Ltd
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Lai Kay Medical Equipment (Beijing) Co.
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 Ltd
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Jinan Zhongkangshun Medical Device Co.
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 Ltd
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.12 Sichuan Yangtze River Medical Device Co.
- 11.2.12.1. Overview
- 11.2.12.2. Products
- 11.2.12.3. SWOT Analysis
- 11.2.12.4. Recent Developments
- 11.2.12.5. Financials (Based on Availability)
- 11.2.13 Ltd
- 11.2.13.1. Overview
- 11.2.13.2. Products
- 11.2.13.3. SWOT Analysis
- 11.2.13.4. Recent Developments
- 11.2.13.5. Financials (Based on Availability)
- 11.2.14 Zhejiang Medical High Medical Technology Co.
- 11.2.14.1. Overview
- 11.2.14.2. Products
- 11.2.14.3. SWOT Analysis
- 11.2.14.4. Recent Developments
- 11.2.14.5. Financials (Based on Availability)
- 11.2.15 Ltd
- 11.2.15.1. Overview
- 11.2.15.2. Products
- 11.2.15.3. SWOT Analysis
- 11.2.15.4. Recent Developments
- 11.2.15.5. Financials (Based on Availability)
- 11.2.16 Shanghai Zhoukang Medical Device Co.
- 11.2.16.1. Overview
- 11.2.16.2. Products
- 11.2.16.3. SWOT Analysis
- 11.2.16.4. Recent Developments
- 11.2.16.5. Financials (Based on Availability)
- 11.2.17 Ltd
- 11.2.17.1. Overview
- 11.2.17.2. Products
- 11.2.17.3. SWOT Analysis
- 11.2.17.4. Recent Developments
- 11.2.17.5. Financials (Based on Availability)
- 11.2.18 Anhui Aerospace Biotechnology Co.
- 11.2.18.1. Overview
- 11.2.18.2. Products
- 11.2.18.3. SWOT Analysis
- 11.2.18.4. Recent Developments
- 11.2.18.5. Financials (Based on Availability)
- 11.2.19 Ltd
- 11.2.19.1. Overview
- 11.2.19.2. Products
- 11.2.19.3. SWOT Analysis
- 11.2.19.4. Recent Developments
- 11.2.19.5. Financials (Based on Availability)
- 11.2.20 Chengdu Weixin Electronic Science and Technology University New Technology Co.
- 11.2.20.1. Overview
- 11.2.20.2. Products
- 11.2.20.3. SWOT Analysis
- 11.2.20.4. Recent Developments
- 11.2.20.5. Financials (Based on Availability)
- 11.2.21 Ltd
- 11.2.21.1. Overview
- 11.2.21.2. Products
- 11.2.21.3. SWOT Analysis
- 11.2.21.4. Recent Developments
- 11.2.21.5. Financials (Based on Availability)
- 11.2.22 MicroPort Youtong Medical Technology (Jiaxing) Co.
- 11.2.22.1. Overview
- 11.2.22.2. Products
- 11.2.22.3. SWOT Analysis
- 11.2.22.4. Recent Developments
- 11.2.22.5. Financials (Based on Availability)
- 11.2.23 Ltd
- 11.2.23.1. Overview
- 11.2.23.2. Products
- 11.2.23.3. SWOT Analysis
- 11.2.23.4. Recent Developments
- 11.2.23.5. Financials (Based on Availability)
- 11.2.24 Shanghai Innova Medical Device Co.
- 11.2.24.1. Overview
- 11.2.24.2. Products
- 11.2.24.3. SWOT Analysis
- 11.2.24.4. Recent Developments
- 11.2.24.5. Financials (Based on Availability)
- 11.2.25 Ltd
- 11.2.25.1. Overview
- 11.2.25.2. Products
- 11.2.25.3. SWOT Analysis
- 11.2.25.4. Recent Developments
- 11.2.25.5. Financials (Based on Availability)
- 11.2.26 Dalian Kuliaite Medical Products Co.
- 11.2.26.1. Overview
- 11.2.26.2. Products
- 11.2.26.3. SWOT Analysis
- 11.2.26.4. Recent Developments
- 11.2.26.5. Financials (Based on Availability)
- 11.2.27 Ltd
- 11.2.27.1. Overview
- 11.2.27.2. Products
- 11.2.27.3. SWOT Analysis
- 11.2.27.4. Recent Developments
- 11.2.27.5. Financials (Based on Availability)
- 11.2.28 Zhuzhou Ruibang Medical Equipment Products Co.
- 11.2.28.1. Overview
- 11.2.28.2. Products
- 11.2.28.3. SWOT Analysis
- 11.2.28.4. Recent Developments
- 11.2.28.5. Financials (Based on Availability)
- 11.2.29 Ltd
- 11.2.29.1. Overview
- 11.2.29.2. Products
- 11.2.29.3. SWOT Analysis
- 11.2.29.4. Recent Developments
- 11.2.29.5. Financials (Based on Availability)
- 11.2.30 Hunan Ruibang Medical Technology Development Co.
- 11.2.30.1. Overview
- 11.2.30.2. Products
- 11.2.30.3. SWOT Analysis
- 11.2.30.4. Recent Developments
- 11.2.30.5. Financials (Based on Availability)
- 11.2.31 Ltd
- 11.2.31.1. Overview
- 11.2.31.2. Products
- 11.2.31.3. SWOT Analysis
- 11.2.31.4. Recent Developments
- 11.2.31.5. Financials (Based on Availability)
- 11.2.1 SRS Medical
List of Figures
- Figure 1: Global Urological Double-j Stent Revenue Breakdown (undefined, %) by Region 2025 & 2033
- Figure 2: North America Urological Double-j Stent Revenue (undefined), by Application 2025 & 2033
- Figure 3: North America Urological Double-j Stent Revenue Share (%), by Application 2025 & 2033
- Figure 4: North America Urological Double-j Stent Revenue (undefined), by Types 2025 & 2033
- Figure 5: North America Urological Double-j Stent Revenue Share (%), by Types 2025 & 2033
- Figure 6: North America Urological Double-j Stent Revenue (undefined), by Country 2025 & 2033
- Figure 7: North America Urological Double-j Stent Revenue Share (%), by Country 2025 & 2033
- Figure 8: South America Urological Double-j Stent Revenue (undefined), by Application 2025 & 2033
- Figure 9: South America Urological Double-j Stent Revenue Share (%), by Application 2025 & 2033
- Figure 10: South America Urological Double-j Stent Revenue (undefined), by Types 2025 & 2033
- Figure 11: South America Urological Double-j Stent Revenue Share (%), by Types 2025 & 2033
- Figure 12: South America Urological Double-j Stent Revenue (undefined), by Country 2025 & 2033
- Figure 13: South America Urological Double-j Stent Revenue Share (%), by Country 2025 & 2033
- Figure 14: Europe Urological Double-j Stent Revenue (undefined), by Application 2025 & 2033
- Figure 15: Europe Urological Double-j Stent Revenue Share (%), by Application 2025 & 2033
- Figure 16: Europe Urological Double-j Stent Revenue (undefined), by Types 2025 & 2033
- Figure 17: Europe Urological Double-j Stent Revenue Share (%), by Types 2025 & 2033
- Figure 18: Europe Urological Double-j Stent Revenue (undefined), by Country 2025 & 2033
- Figure 19: Europe Urological Double-j Stent Revenue Share (%), by Country 2025 & 2033
- Figure 20: Middle East & Africa Urological Double-j Stent Revenue (undefined), by Application 2025 & 2033
- Figure 21: Middle East & Africa Urological Double-j Stent Revenue Share (%), by Application 2025 & 2033
- Figure 22: Middle East & Africa Urological Double-j Stent Revenue (undefined), by Types 2025 & 2033
- Figure 23: Middle East & Africa Urological Double-j Stent Revenue Share (%), by Types 2025 & 2033
- Figure 24: Middle East & Africa Urological Double-j Stent Revenue (undefined), by Country 2025 & 2033
- Figure 25: Middle East & Africa Urological Double-j Stent Revenue Share (%), by Country 2025 & 2033
- Figure 26: Asia Pacific Urological Double-j Stent Revenue (undefined), by Application 2025 & 2033
- Figure 27: Asia Pacific Urological Double-j Stent Revenue Share (%), by Application 2025 & 2033
- Figure 28: Asia Pacific Urological Double-j Stent Revenue (undefined), by Types 2025 & 2033
- Figure 29: Asia Pacific Urological Double-j Stent Revenue Share (%), by Types 2025 & 2033
- Figure 30: Asia Pacific Urological Double-j Stent Revenue (undefined), by Country 2025 & 2033
- Figure 31: Asia Pacific Urological Double-j Stent Revenue Share (%), by Country 2025 & 2033
List of Tables
- Table 1: Global Urological Double-j Stent Revenue undefined Forecast, by Application 2020 & 2033
- Table 2: Global Urological Double-j Stent Revenue undefined Forecast, by Types 2020 & 2033
- Table 3: Global Urological Double-j Stent Revenue undefined Forecast, by Region 2020 & 2033
- Table 4: Global Urological Double-j Stent Revenue undefined Forecast, by Application 2020 & 2033
- Table 5: Global Urological Double-j Stent Revenue undefined Forecast, by Types 2020 & 2033
- Table 6: Global Urological Double-j Stent Revenue undefined Forecast, by Country 2020 & 2033
- Table 7: United States Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 8: Canada Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 9: Mexico Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 10: Global Urological Double-j Stent Revenue undefined Forecast, by Application 2020 & 2033
- Table 11: Global Urological Double-j Stent Revenue undefined Forecast, by Types 2020 & 2033
- Table 12: Global Urological Double-j Stent Revenue undefined Forecast, by Country 2020 & 2033
- Table 13: Brazil Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 14: Argentina Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 15: Rest of South America Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 16: Global Urological Double-j Stent Revenue undefined Forecast, by Application 2020 & 2033
- Table 17: Global Urological Double-j Stent Revenue undefined Forecast, by Types 2020 & 2033
- Table 18: Global Urological Double-j Stent Revenue undefined Forecast, by Country 2020 & 2033
- Table 19: United Kingdom Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 20: Germany Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 21: France Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 22: Italy Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 23: Spain Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 24: Russia Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 25: Benelux Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 26: Nordics Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 27: Rest of Europe Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 28: Global Urological Double-j Stent Revenue undefined Forecast, by Application 2020 & 2033
- Table 29: Global Urological Double-j Stent Revenue undefined Forecast, by Types 2020 & 2033
- Table 30: Global Urological Double-j Stent Revenue undefined Forecast, by Country 2020 & 2033
- Table 31: Turkey Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 32: Israel Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 33: GCC Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 34: North Africa Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 35: South Africa Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 36: Rest of Middle East & Africa Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 37: Global Urological Double-j Stent Revenue undefined Forecast, by Application 2020 & 2033
- Table 38: Global Urological Double-j Stent Revenue undefined Forecast, by Types 2020 & 2033
- Table 39: Global Urological Double-j Stent Revenue undefined Forecast, by Country 2020 & 2033
- Table 40: China Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 41: India Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 42: Japan Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 43: South Korea Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 44: ASEAN Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 45: Oceania Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
- Table 46: Rest of Asia Pacific Urological Double-j Stent Revenue (undefined) Forecast, by Application 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Urological Double-j Stent?
The projected CAGR is approximately 6.96%.
2. Which companies are prominent players in the Urological Double-j Stent?
Key companies in the market include SRS Medical, Boston Scientific, Bard Medical, CR Bard, MDS, Zhejiang Ruiding Biotechnology Co., Ltd, Lai Kay Medical Equipment (Beijing) Co., Ltd, Jinan Zhongkangshun Medical Device Co., Ltd, Sichuan Yangtze River Medical Device Co., Ltd, Zhejiang Medical High Medical Technology Co., Ltd, Shanghai Zhoukang Medical Device Co., Ltd, Anhui Aerospace Biotechnology Co., Ltd, Chengdu Weixin Electronic Science and Technology University New Technology Co., Ltd, MicroPort Youtong Medical Technology (Jiaxing) Co., Ltd, Shanghai Innova Medical Device Co., Ltd, Dalian Kuliaite Medical Products Co., Ltd, Zhuzhou Ruibang Medical Equipment Products Co., Ltd, Hunan Ruibang Medical Technology Development Co., Ltd.
3. What are the main segments of the Urological Double-j Stent?
The market segments include Application, Types.
4. Can you provide details about the market size?
The market size is estimated to be USD XXX N/A as of 2022.
5. What are some drivers contributing to market growth?
N/A
6. What are the notable trends driving market growth?
N/A
7. Are there any restraints impacting market growth?
N/A
8. Can you provide examples of recent developments in the market?
N/A
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 2900.00, USD 4350.00, and USD 5800.00 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in N/A.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Urological Double-j Stent," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Urological Double-j Stent report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Urological Double-j Stent?
To stay informed about further developments, trends, and reports in the Urological Double-j Stent, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database
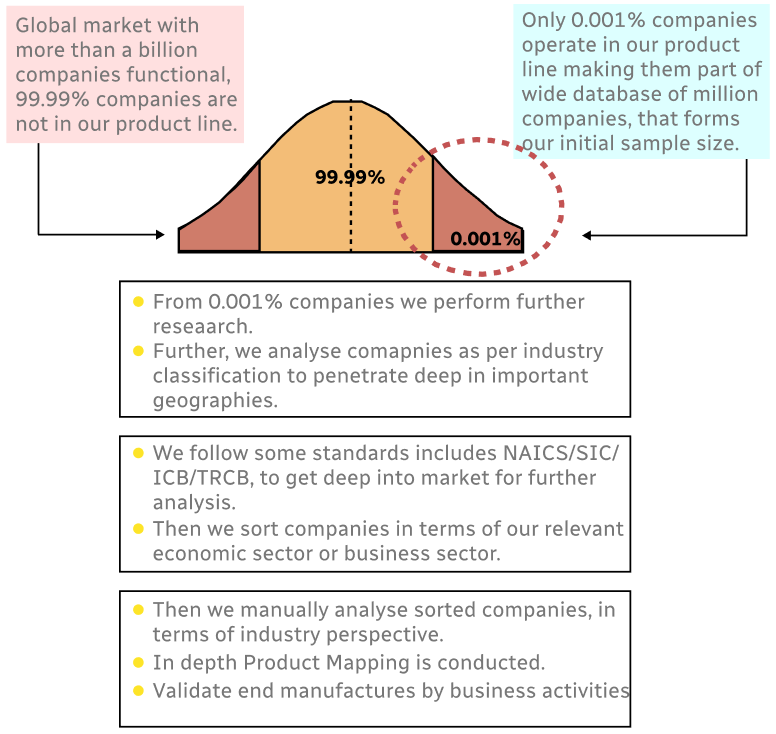
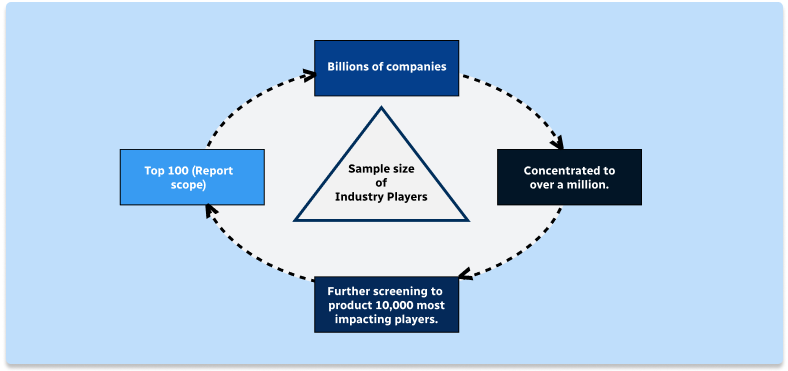
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
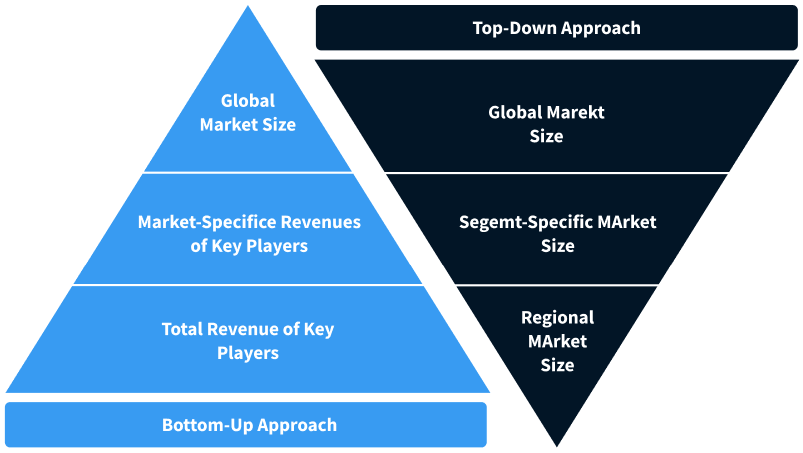
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
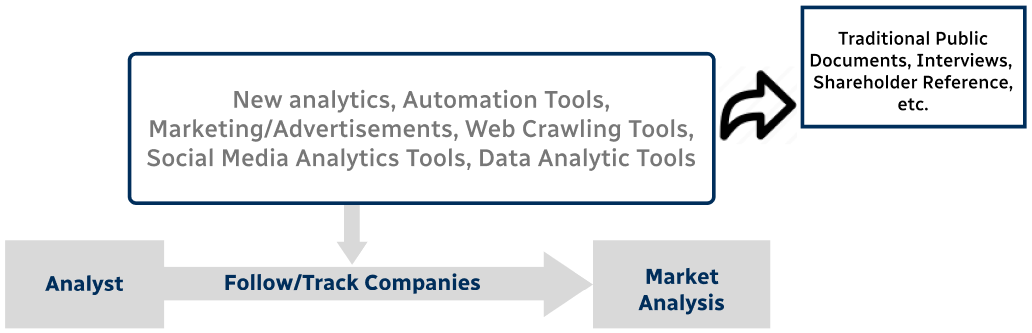
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


