Key Insights
The global Non-invasive Prenatal Testing (NIPT) market is set for significant growth, driven by increased awareness of fetal genetic abnormalities and a rising preference for safer, non-invasive diagnostic methods. The market is projected to reach $6.28 billion by 2025, with a Compound Annual Growth Rate (CAGR) of 14.9% through 2033. This expansion is propelled by advancements in next-generation sequencing (NGS) technology, enhancing NIPT accuracy and accessibility. Key growth factors include rising maternal age, higher demand for early detection of conditions like Down Syndrome, Edwards Syndrome, and Patau Syndrome, and favorable reimbursement policies. The proliferation of diagnostic laboratories and increasing NIPT adoption in emerging economies further bolster market momentum.
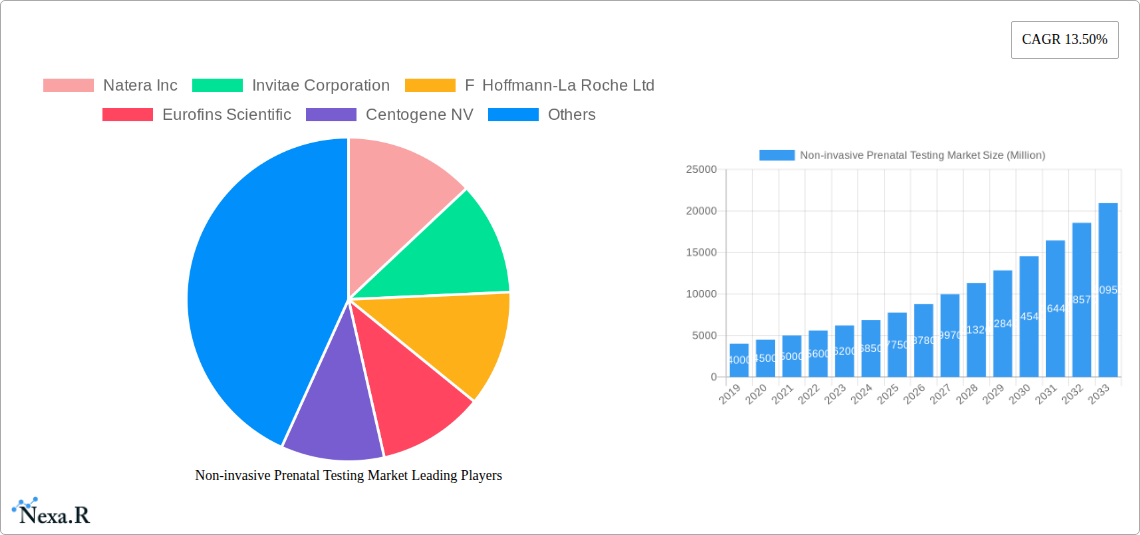
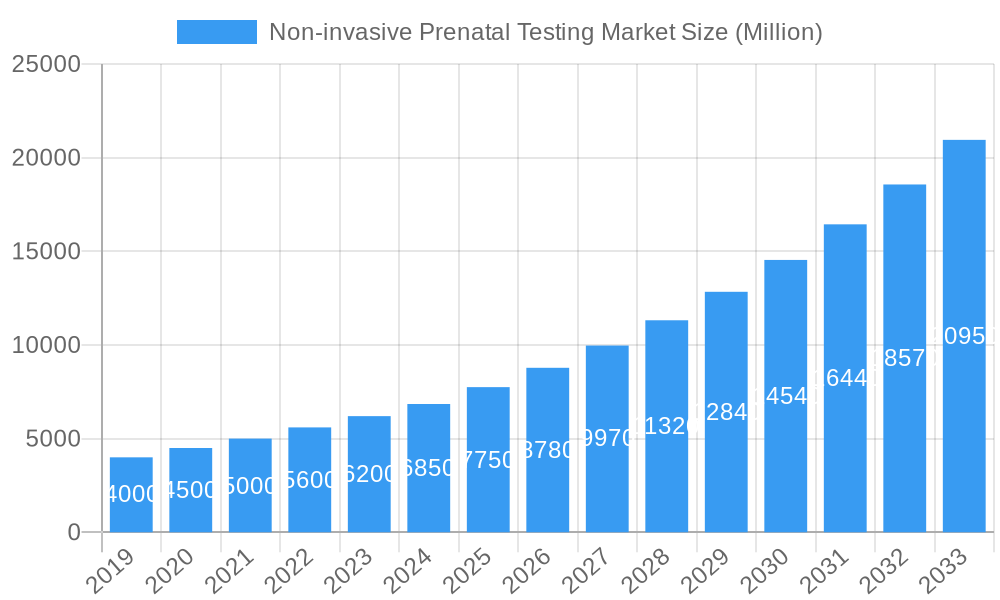
Non-invasive Prenatal Testing Market Market Size (In Billion)

The NIPT market comprises Instruments, Kits and Reagents, and Services. Screening for common aneuploidies, particularly Down Syndrome (trisomy 21), Edwards Syndrome (trisomy 18), and Patau Syndrome (trisomy 13), dominates the application segment. Turner Syndrome and other rare genetic disorders are also gaining focus. Hospitals and Diagnostic Labs are the primary end-users, increasingly integrating NIPT into routine prenatal care. North America and Europe currently lead the market due to established healthcare infrastructures and high adoption rates. However, the Asia Pacific region is anticipated to experience the fastest growth, fueled by a large population, rising disposable incomes, and an escalating focus on reproductive health. Leading companies such as Natera Inc., Invitae Corporation, and F. Hoffmann-La Roche Ltd. are actively engaged in research, product launches, and strategic collaborations to enhance their market presence.
Non-invasive Prenatal Testing Market Company Market Share

Gain essential insights into the dynamic Non-invasive Prenatal Testing (NIPT) market. This comprehensive report, covering 2019-2033 with a base and estimated year of 2025, offers a strategic guide for stakeholders in prenatal diagnostics. Analyze market trends, growth trajectories, regional leadership, product innovations, key players, and emerging opportunities to inform your business strategies.
Non-invasive Prenatal Testing Market Market Dynamics & Structure
The Non-invasive Prenatal Testing (NIPT) market is characterized by a moderate to high concentration, driven by significant technological advancements and increasing awareness among expectant parents and healthcare providers. Key drivers of innovation include the continuous refinement of next-generation sequencing (NGS) technologies, advancements in bioinformatics for more accurate data analysis, and the development of more sensitive and specific assay chemistries. Regulatory frameworks, particularly in North America and Europe, are evolving to accommodate the growing utility of NIPT, influencing market access and reimbursement policies. Competitive product substitutes, such as traditional prenatal screening methods (e.g., maternal serum screening, ultrasound), still exist but are increasingly being supplanted by NIPT's superior accuracy and non-invasive nature. End-user demographics are expanding beyond high-risk pregnancies to include routine screening for all pregnancies, fueled by improving cost-effectiveness and accessibility. Mergers and acquisitions (M&A) are a notable trend as larger players aim to consolidate market share, acquire innovative technologies, and expand their geographic reach.
- Technological Innovation Drivers: Miniaturization of sequencing platforms, AI-powered data interpretation, enhanced single-nucleotide polymorphism (SNP) based NIPT, and improved algorithms for low fetal fraction detection.
- Regulatory Frameworks: FDA approvals for direct-to-consumer NIPT, CLIA certification for laboratories, and evolving reimbursement landscapes across different healthcare systems.
- Competitive Product Substitutes: Maternal serum screening, ultrasound-based aneuploidy screening, and invasive diagnostic tests (amniocentesis, CVS) for confirmation.
- End-User Demographics: Expansion from high-risk to average-risk pregnancies, increasing demand for early pregnancy screening, and growing interest in expanded NIPT panels for microdeletion syndromes.
- M&A Trends: Consolidation by major diagnostic companies, acquisition of smaller NIPT technology providers, and strategic partnerships to expand service offerings.
Non-invasive Prenatal Testing Market Growth Trends & Insights
The global Non-invasive Prenatal Testing (NIPT) market is poised for robust growth, driven by an escalating adoption rate fueled by its unparalleled accuracy, safety, and expanding clinical utility. The market size is projected to witness a significant expansion from an estimated XX Million in 2024 to XX Million by 2033, reflecting a compound annual growth rate (CAGR) of approximately 12.5% during the forecast period (2025-2033). This upward trajectory is propelled by several converging factors, including increasing awareness among expectant parents about the benefits of early and accurate genetic screening, a growing preference for non-invasive diagnostic methods over traditional invasive procedures like amniocentesis, and supportive government initiatives aimed at improving maternal and child health outcomes. Technological disruptions, particularly in the realm of next-generation sequencing (NGS) and advanced bioinformatics, are continuously enhancing the precision and scope of NIPT, enabling the detection of a wider range of chromosomal abnormalities and genetic conditions.
Consumer behavior shifts are also playing a pivotal role. Expectant parents are becoming more proactive in seeking comprehensive prenatal information, leading to a higher demand for advanced screening options. The declining cost of sequencing technologies, coupled with expanding insurance coverage and reimbursement policies in various regions, is further democratizing access to NIPT, making it more accessible to a broader population. The penetration of NIPT in average-risk pregnancies is steadily increasing, moving beyond its initial application in high-risk pregnancies. This broader adoption is a key indicator of market maturity and consumer trust. Furthermore, the development of expanded NIPT panels that screen for microdeletion syndromes and copy number variations is significantly broadening the application spectrum of NIPT, thereby driving market growth. The integration of NIPT results with other prenatal screening modalities and the increasing use of advanced analytical tools for interpreting complex genetic data are also contributing to the market's dynamism.
Dominant Regions, Countries, or Segments in Non-invasive Prenatal Testing Market
North America, particularly the United States, currently dominates the Non-invasive Prenatal Testing (NIPT) market. This regional supremacy is attributable to several interconnected factors, including a well-established healthcare infrastructure, high healthcare expenditure, strong government support for advanced diagnostic technologies, and a high level of consumer awareness regarding genetic screening. The presence of leading NIPT manufacturers and research institutions in the region fosters continuous innovation and rapid adoption of new technologies. Furthermore, favorable reimbursement policies from private payers and increasing Medicare coverage for NIPT in specific high-risk pregnancies have significantly boosted its accessibility and utilization.
Within North America, the United States accounts for the largest market share, driven by a proactive approach to prenatal care, a large patient pool, and significant investment in R&D by companies like Natera Inc. and Invitae Corporation. The widespread availability of advanced diagnostic laboratories and hospitals equipped with state-of-the-art sequencing technologies further solidifies its leading position.
In terms of segments, Kits and Reagents currently represent the largest and fastest-growing component within the NIPT market. This dominance is due to the high demand for consumables associated with the widespread adoption of NIPT assays. The increasing number of NIPT tests being performed globally directly translates into a higher consumption of specialized kits and reagents required for sample processing, library preparation, and sequencing.
Regarding applications, Down Syndrome (trisomy 21) remains the most prevalent application, given its high incidence and the established efficacy of NIPT in its detection. However, the market is witnessing a rapid expansion in the adoption of NIPT for other trisomies, such as Edwards Syndrome (trisomy 18) and Patau Syndrome (trisomy 13), as well as for sex chromosome aneuploidies like Turner Syndrome. The growing demand for comprehensive screening leading to the detection of Other Applications, including microdeletion syndromes, is also contributing significantly to market growth.
The Diagnostic Labs segment is also a major contributor to market growth, as these facilities are at the forefront of performing NIPT tests. The increasing number of specialized genetic testing laboratories and the expansion of in-house NIPT capabilities within larger hospital networks are key drivers.
- Key Drivers in North America: High disposable incomes, advanced healthcare systems, robust R&D investment, established regulatory pathways, and increasing patient demand for advanced prenatal diagnostics.
- Dominant Country (North America): United States, owing to its market size, technological leadership, and insurance coverage.
- Dominant Segment (Component): Kits and Reagents, driven by the high volume of NIPT tests performed.
- Dominant Segment (Application): Down Syndrome (trisomy 21), with growing traction for other aneuploidies and microdeletions.
- Dominant Segment (End User): Diagnostic Labs, as the primary service providers.
Non-invasive Prenatal Testing Market Product Landscape
The Non-invasive Prenatal Testing (NIPT) product landscape is characterized by continuous innovation aimed at enhancing accuracy, expanding the scope of detectable genetic abnormalities, and improving user experience. Leading companies are focusing on developing highly sensitive and specific assays that can accurately detect aneuploidies and microdeletion syndromes even with low fetal DNA fractions. Innovations include the development of novel bioinformatics algorithms for improved data interpretation, the integration of advanced sequencing technologies for greater throughput and cost-effectiveness, and the introduction of user-friendly platforms that streamline sample processing and reporting. The performance metrics of NIPT products are constantly being refined, with manufacturers striving for higher detection rates and lower false-positive and false-negative rates. Unique selling propositions often revolve around the breadth of conditions screened, the speed of turnaround time, and the quality of clinical support provided.
Key Drivers, Barriers & Challenges in Non-invasive Prenatal Testing Market
Key Drivers:
- Technological Advancements: Continuous improvements in NGS technology and bioinformatics algorithms enhance NIPT accuracy and expand its capabilities.
- Increasing Awareness & Demand: Growing understanding of NIPT benefits among expectant parents and healthcare providers drives adoption.
- Safety and Non-invasiveness: The avoidance of miscarriage risk associated with invasive procedures makes NIPT highly desirable.
- Expanding Reimbursement: Increasing insurance coverage and favorable reimbursement policies broaden accessibility.
- Cost-Effectiveness: Declining sequencing costs make NIPT more affordable for a wider population.
Barriers & Challenges:
- Regulatory Hurdles: Navigating complex and varying regulatory approvals across different countries can be challenging.
- Reimbursement Variations: Inconsistent reimbursement policies in certain regions limit accessibility and adoption.
- Interpretation of Results: Managing complex genetic data and ensuring appropriate genetic counseling for nuanced results remains a challenge.
- Limited Availability in Developing Regions: Lack of advanced infrastructure and trained personnel in some developing countries hinders widespread adoption.
- Ethical Considerations: Ongoing debates surrounding the ethical implications of genetic screening and potential for selective abortion.
Emerging Opportunities in Non-invasive Prenatal Testing Market
Emerging opportunities in the Non-invasive Prenatal Testing (NIPT) market lie in the expansion of screening panels to include a wider array of rare genetic disorders and single-gene conditions. The development of cost-effective NIPT solutions for low- and middle-income countries presents a significant untapped market. Furthermore, the integration of NIPT with other advanced prenatal diagnostic tools, such as liquid biopsy for cancer detection in pregnant women, offers novel application areas. The growing consumer demand for personalized genetic information and early disease detection is also driving opportunities for direct-to-consumer NIPT services, with robust genetic counseling support.
Growth Accelerators in the Non-invasive Prenatal Testing Market Industry
The Non-invasive Prenatal Testing (NIPT) market is witnessing accelerated growth fueled by ongoing technological breakthroughs in ultra-high-throughput sequencing and AI-driven diagnostic interpretation. Strategic partnerships between diagnostic technology providers, clinical laboratories, and healthcare systems are crucial in expanding market reach and enhancing service delivery. Market expansion strategies, including the development of localized NIPT solutions tailored to specific regional needs and regulatory landscapes, are further propelling growth. The increasing focus on proactive prenatal care and the shift towards personalized medicine are creating a fertile ground for NIPT to become a standard of care globally.
Key Players Shaping the Non-invasive Prenatal Testing Market Market
- Natera Inc.
- Invitae Corporation
- F Hoffmann-La Roche Ltd
- Eurofins Scientific
- Centogene NV
- Myriad Womens Health Inc
- BGI
- Qiagen
- PerkinElmer Inc.
- Illumina Inc.
- MedGenome Labs Ltd
Notable Milestones in Non-invasive Prenatal Testing Market Sector
- August 2022: Natera Inc. filed a pre-submission to the Food and Drug Administration (FDA) for its panorama non-invasive prenatal test (NIPT) as part of the Q-Sub process. The company filed its pre-submission in June 2022 for fetal chromosomal aneuploidies and 22q11.2 deletion syndrome.
- July 2022: Genetic Technologies Limited acquired EasyDNA as the company expanded the availability on its websites of Carrier Testing and Non-Invasive Prenatal Tests (NIPT) in Europe.
In-Depth Non-invasive Prenatal Testing Market Market Outlook
The future outlook for the Non-invasive Prenatal Testing (NIPT) market is exceptionally promising, driven by an anticipated surge in demand for comprehensive and accurate prenatal diagnostics. Growth accelerators such as advancements in liquid biopsy techniques, expanding the scope beyond aneuploidies to include rare genetic disorders and fetal cell-free DNA (cfDNA) analysis for infectious diseases, are set to redefine the prenatal screening landscape. Strategic collaborations aimed at improving NIPT accessibility in underserved regions and the development of integrated genetic counseling platforms will be pivotal. The increasing integration of NIPT with advanced genomics and AI-powered predictive analytics will further solidify its position as an indispensable tool in modern obstetrics and gynecology, promising a healthier future for expectant parents and newborns.
Non-invasive Prenatal Testing Market Segmentation
-
1. Component
- 1.1. Instruments
- 1.2. Kits and Reagents
- 1.3. Services
-
2. Application
- 2.1. Down Syndrome (trisomy 21)
- 2.2. Edwards Syndrome (trisomy 18)
- 2.3. Patau Syndrome (trisomy 13)
- 2.4. Turner Syndrome
- 2.5. Other Applications
-
3. End User
- 3.1. Hospitals
- 3.2. Diagnostic Labs
Non-invasive Prenatal Testing Market Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. Europe
- 2.1. Germany
- 2.2. United kingdom
- 2.3. France
- 2.4. Italy
- 2.5. Spain
- 2.6. Rest of Europe
-
3. Asia Pacific
- 3.1. China
- 3.2. Japan
- 3.3. India
- 3.4. Australia
- 3.5. South Korea
- 3.6. Rest of Asia Pacific
-
4. Middle East and Africa
- 4.1. GCC
- 4.2. South Africa
- 4.3. Rest of Middle East and Africa
-
5. South America
- 5.1. Brazil
- 5.2. Argentina
- 5.3. Rest of South America
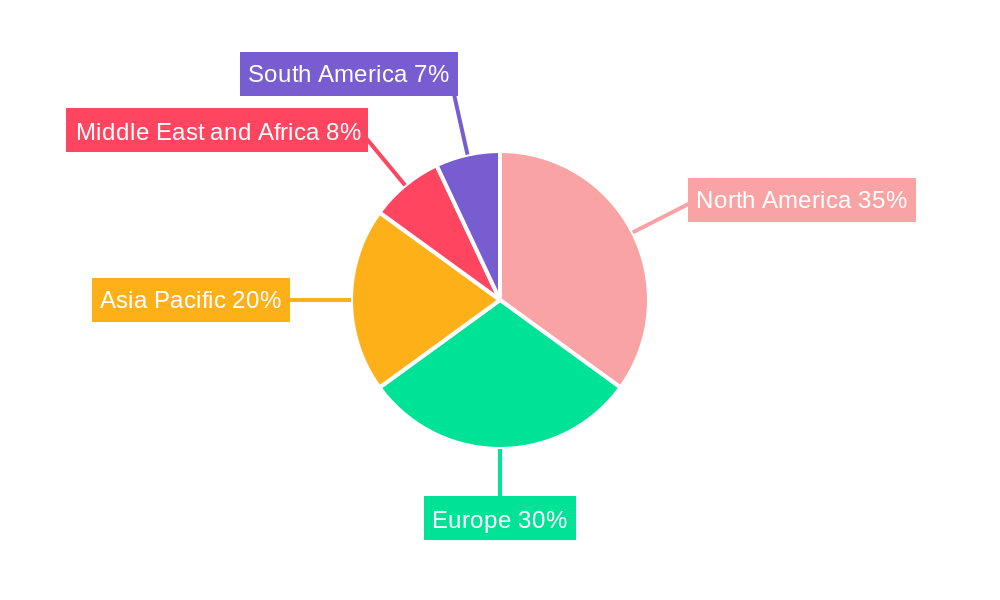
Non-invasive Prenatal Testing Market Regional Market Share

Geographic Coverage of Non-invasive Prenatal Testing Market
Non-invasive Prenatal Testing Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 14.9% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Number of Babies with Chromosomal Disorders Owing to Increasing Number of Late Pregnancies; Increasing Demand for Early and Non-invasive Fetal Diagnosis; Favorable Reimbursement Policies
- 3.3. Market Restrains
- 3.3.1. Lack of Skilled Professionals; Stringent Regulations and Ethical Concerns
- 3.4. Market Trends
- 3.4.1. Down Syndrome Segment Dominates the Non-invasive Prenatal Testing Market
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Non-invasive Prenatal Testing Market Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Component
- 5.1.1. Instruments
- 5.1.2. Kits and Reagents
- 5.1.3. Services
- 5.2. Market Analysis, Insights and Forecast - by Application
- 5.2.1. Down Syndrome (trisomy 21)
- 5.2.2. Edwards Syndrome (trisomy 18)
- 5.2.3. Patau Syndrome (trisomy 13)
- 5.2.4. Turner Syndrome
- 5.2.5. Other Applications
- 5.3. Market Analysis, Insights and Forecast - by End User
- 5.3.1. Hospitals
- 5.3.2. Diagnostic Labs
- 5.4. Market Analysis, Insights and Forecast - by Region
- 5.4.1. North America
- 5.4.2. Europe
- 5.4.3. Asia Pacific
- 5.4.4. Middle East and Africa
- 5.4.5. South America
- 5.1. Market Analysis, Insights and Forecast - by Component
- 6. North America Non-invasive Prenatal Testing Market Analysis, Insights and Forecast, 2020-2032
- 6.1. Market Analysis, Insights and Forecast - by Component
- 6.1.1. Instruments
- 6.1.2. Kits and Reagents
- 6.1.3. Services
- 6.2. Market Analysis, Insights and Forecast - by Application
- 6.2.1. Down Syndrome (trisomy 21)
- 6.2.2. Edwards Syndrome (trisomy 18)
- 6.2.3. Patau Syndrome (trisomy 13)
- 6.2.4. Turner Syndrome
- 6.2.5. Other Applications
- 6.3. Market Analysis, Insights and Forecast - by End User
- 6.3.1. Hospitals
- 6.3.2. Diagnostic Labs
- 6.1. Market Analysis, Insights and Forecast - by Component
- 7. Europe Non-invasive Prenatal Testing Market Analysis, Insights and Forecast, 2020-2032
- 7.1. Market Analysis, Insights and Forecast - by Component
- 7.1.1. Instruments
- 7.1.2. Kits and Reagents
- 7.1.3. Services
- 7.2. Market Analysis, Insights and Forecast - by Application
- 7.2.1. Down Syndrome (trisomy 21)
- 7.2.2. Edwards Syndrome (trisomy 18)
- 7.2.3. Patau Syndrome (trisomy 13)
- 7.2.4. Turner Syndrome
- 7.2.5. Other Applications
- 7.3. Market Analysis, Insights and Forecast - by End User
- 7.3.1. Hospitals
- 7.3.2. Diagnostic Labs
- 7.1. Market Analysis, Insights and Forecast - by Component
- 8. Asia Pacific Non-invasive Prenatal Testing Market Analysis, Insights and Forecast, 2020-2032
- 8.1. Market Analysis, Insights and Forecast - by Component
- 8.1.1. Instruments
- 8.1.2. Kits and Reagents
- 8.1.3. Services
- 8.2. Market Analysis, Insights and Forecast - by Application
- 8.2.1. Down Syndrome (trisomy 21)
- 8.2.2. Edwards Syndrome (trisomy 18)
- 8.2.3. Patau Syndrome (trisomy 13)
- 8.2.4. Turner Syndrome
- 8.2.5. Other Applications
- 8.3. Market Analysis, Insights and Forecast - by End User
- 8.3.1. Hospitals
- 8.3.2. Diagnostic Labs
- 8.1. Market Analysis, Insights and Forecast - by Component
- 9. Middle East and Africa Non-invasive Prenatal Testing Market Analysis, Insights and Forecast, 2020-2032
- 9.1. Market Analysis, Insights and Forecast - by Component
- 9.1.1. Instruments
- 9.1.2. Kits and Reagents
- 9.1.3. Services
- 9.2. Market Analysis, Insights and Forecast - by Application
- 9.2.1. Down Syndrome (trisomy 21)
- 9.2.2. Edwards Syndrome (trisomy 18)
- 9.2.3. Patau Syndrome (trisomy 13)
- 9.2.4. Turner Syndrome
- 9.2.5. Other Applications
- 9.3. Market Analysis, Insights and Forecast - by End User
- 9.3.1. Hospitals
- 9.3.2. Diagnostic Labs
- 9.1. Market Analysis, Insights and Forecast - by Component
- 10. South America Non-invasive Prenatal Testing Market Analysis, Insights and Forecast, 2020-2032
- 10.1. Market Analysis, Insights and Forecast - by Component
- 10.1.1. Instruments
- 10.1.2. Kits and Reagents
- 10.1.3. Services
- 10.2. Market Analysis, Insights and Forecast - by Application
- 10.2.1. Down Syndrome (trisomy 21)
- 10.2.2. Edwards Syndrome (trisomy 18)
- 10.2.3. Patau Syndrome (trisomy 13)
- 10.2.4. Turner Syndrome
- 10.2.5. Other Applications
- 10.3. Market Analysis, Insights and Forecast - by End User
- 10.3.1. Hospitals
- 10.3.2. Diagnostic Labs
- 10.1. Market Analysis, Insights and Forecast - by Component
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2025
- 11.2. Company Profiles
- 11.2.1 Natera Inc
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 Invitae Corporation
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 F Hoffmann-La Roche Ltd
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Eurofins Scientific
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 Centogene NV
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Myriad Womens Health Inc
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 BGI
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Qiagen*List Not Exhaustive
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 PerkinElmer Inc
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Illumina Inc
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 MedGenome Labs Ltd
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.1 Natera Inc
List of Figures
- Figure 1: Global Non-invasive Prenatal Testing Market Revenue Breakdown (billion, %) by Region 2025 & 2033
- Figure 2: North America Non-invasive Prenatal Testing Market Revenue (billion), by Component 2025 & 2033
- Figure 3: North America Non-invasive Prenatal Testing Market Revenue Share (%), by Component 2025 & 2033
- Figure 4: North America Non-invasive Prenatal Testing Market Revenue (billion), by Application 2025 & 2033
- Figure 5: North America Non-invasive Prenatal Testing Market Revenue Share (%), by Application 2025 & 2033
- Figure 6: North America Non-invasive Prenatal Testing Market Revenue (billion), by End User 2025 & 2033
- Figure 7: North America Non-invasive Prenatal Testing Market Revenue Share (%), by End User 2025 & 2033
- Figure 8: North America Non-invasive Prenatal Testing Market Revenue (billion), by Country 2025 & 2033
- Figure 9: North America Non-invasive Prenatal Testing Market Revenue Share (%), by Country 2025 & 2033
- Figure 10: Europe Non-invasive Prenatal Testing Market Revenue (billion), by Component 2025 & 2033
- Figure 11: Europe Non-invasive Prenatal Testing Market Revenue Share (%), by Component 2025 & 2033
- Figure 12: Europe Non-invasive Prenatal Testing Market Revenue (billion), by Application 2025 & 2033
- Figure 13: Europe Non-invasive Prenatal Testing Market Revenue Share (%), by Application 2025 & 2033
- Figure 14: Europe Non-invasive Prenatal Testing Market Revenue (billion), by End User 2025 & 2033
- Figure 15: Europe Non-invasive Prenatal Testing Market Revenue Share (%), by End User 2025 & 2033
- Figure 16: Europe Non-invasive Prenatal Testing Market Revenue (billion), by Country 2025 & 2033
- Figure 17: Europe Non-invasive Prenatal Testing Market Revenue Share (%), by Country 2025 & 2033
- Figure 18: Asia Pacific Non-invasive Prenatal Testing Market Revenue (billion), by Component 2025 & 2033
- Figure 19: Asia Pacific Non-invasive Prenatal Testing Market Revenue Share (%), by Component 2025 & 2033
- Figure 20: Asia Pacific Non-invasive Prenatal Testing Market Revenue (billion), by Application 2025 & 2033
- Figure 21: Asia Pacific Non-invasive Prenatal Testing Market Revenue Share (%), by Application 2025 & 2033
- Figure 22: Asia Pacific Non-invasive Prenatal Testing Market Revenue (billion), by End User 2025 & 2033
- Figure 23: Asia Pacific Non-invasive Prenatal Testing Market Revenue Share (%), by End User 2025 & 2033
- Figure 24: Asia Pacific Non-invasive Prenatal Testing Market Revenue (billion), by Country 2025 & 2033
- Figure 25: Asia Pacific Non-invasive Prenatal Testing Market Revenue Share (%), by Country 2025 & 2033
- Figure 26: Middle East and Africa Non-invasive Prenatal Testing Market Revenue (billion), by Component 2025 & 2033
- Figure 27: Middle East and Africa Non-invasive Prenatal Testing Market Revenue Share (%), by Component 2025 & 2033
- Figure 28: Middle East and Africa Non-invasive Prenatal Testing Market Revenue (billion), by Application 2025 & 2033
- Figure 29: Middle East and Africa Non-invasive Prenatal Testing Market Revenue Share (%), by Application 2025 & 2033
- Figure 30: Middle East and Africa Non-invasive Prenatal Testing Market Revenue (billion), by End User 2025 & 2033
- Figure 31: Middle East and Africa Non-invasive Prenatal Testing Market Revenue Share (%), by End User 2025 & 2033
- Figure 32: Middle East and Africa Non-invasive Prenatal Testing Market Revenue (billion), by Country 2025 & 2033
- Figure 33: Middle East and Africa Non-invasive Prenatal Testing Market Revenue Share (%), by Country 2025 & 2033
- Figure 34: South America Non-invasive Prenatal Testing Market Revenue (billion), by Component 2025 & 2033
- Figure 35: South America Non-invasive Prenatal Testing Market Revenue Share (%), by Component 2025 & 2033
- Figure 36: South America Non-invasive Prenatal Testing Market Revenue (billion), by Application 2025 & 2033
- Figure 37: South America Non-invasive Prenatal Testing Market Revenue Share (%), by Application 2025 & 2033
- Figure 38: South America Non-invasive Prenatal Testing Market Revenue (billion), by End User 2025 & 2033
- Figure 39: South America Non-invasive Prenatal Testing Market Revenue Share (%), by End User 2025 & 2033
- Figure 40: South America Non-invasive Prenatal Testing Market Revenue (billion), by Country 2025 & 2033
- Figure 41: South America Non-invasive Prenatal Testing Market Revenue Share (%), by Country 2025 & 2033
List of Tables
- Table 1: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by Component 2020 & 2033
- Table 2: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by Application 2020 & 2033
- Table 3: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by End User 2020 & 2033
- Table 4: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by Region 2020 & 2033
- Table 5: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by Component 2020 & 2033
- Table 6: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by Application 2020 & 2033
- Table 7: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by End User 2020 & 2033
- Table 8: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by Country 2020 & 2033
- Table 9: United States Non-invasive Prenatal Testing Market Revenue (billion) Forecast, by Application 2020 & 2033
- Table 10: Canada Non-invasive Prenatal Testing Market Revenue (billion) Forecast, by Application 2020 & 2033
- Table 11: Mexico Non-invasive Prenatal Testing Market Revenue (billion) Forecast, by Application 2020 & 2033
- Table 12: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by Component 2020 & 2033
- Table 13: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by Application 2020 & 2033
- Table 14: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by End User 2020 & 2033
- Table 15: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by Country 2020 & 2033
- Table 16: Germany Non-invasive Prenatal Testing Market Revenue (billion) Forecast, by Application 2020 & 2033
- Table 17: United kingdom Non-invasive Prenatal Testing Market Revenue (billion) Forecast, by Application 2020 & 2033
- Table 18: France Non-invasive Prenatal Testing Market Revenue (billion) Forecast, by Application 2020 & 2033
- Table 19: Italy Non-invasive Prenatal Testing Market Revenue (billion) Forecast, by Application 2020 & 2033
- Table 20: Spain Non-invasive Prenatal Testing Market Revenue (billion) Forecast, by Application 2020 & 2033
- Table 21: Rest of Europe Non-invasive Prenatal Testing Market Revenue (billion) Forecast, by Application 2020 & 2033
- Table 22: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by Component 2020 & 2033
- Table 23: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by Application 2020 & 2033
- Table 24: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by End User 2020 & 2033
- Table 25: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by Country 2020 & 2033
- Table 26: China Non-invasive Prenatal Testing Market Revenue (billion) Forecast, by Application 2020 & 2033
- Table 27: Japan Non-invasive Prenatal Testing Market Revenue (billion) Forecast, by Application 2020 & 2033
- Table 28: India Non-invasive Prenatal Testing Market Revenue (billion) Forecast, by Application 2020 & 2033
- Table 29: Australia Non-invasive Prenatal Testing Market Revenue (billion) Forecast, by Application 2020 & 2033
- Table 30: South Korea Non-invasive Prenatal Testing Market Revenue (billion) Forecast, by Application 2020 & 2033
- Table 31: Rest of Asia Pacific Non-invasive Prenatal Testing Market Revenue (billion) Forecast, by Application 2020 & 2033
- Table 32: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by Component 2020 & 2033
- Table 33: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by Application 2020 & 2033
- Table 34: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by End User 2020 & 2033
- Table 35: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by Country 2020 & 2033
- Table 36: GCC Non-invasive Prenatal Testing Market Revenue (billion) Forecast, by Application 2020 & 2033
- Table 37: South Africa Non-invasive Prenatal Testing Market Revenue (billion) Forecast, by Application 2020 & 2033
- Table 38: Rest of Middle East and Africa Non-invasive Prenatal Testing Market Revenue (billion) Forecast, by Application 2020 & 2033
- Table 39: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by Component 2020 & 2033
- Table 40: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by Application 2020 & 2033
- Table 41: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by End User 2020 & 2033
- Table 42: Global Non-invasive Prenatal Testing Market Revenue billion Forecast, by Country 2020 & 2033
- Table 43: Brazil Non-invasive Prenatal Testing Market Revenue (billion) Forecast, by Application 2020 & 2033
- Table 44: Argentina Non-invasive Prenatal Testing Market Revenue (billion) Forecast, by Application 2020 & 2033
- Table 45: Rest of South America Non-invasive Prenatal Testing Market Revenue (billion) Forecast, by Application 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Non-invasive Prenatal Testing Market?
The projected CAGR is approximately 14.9%.
2. Which companies are prominent players in the Non-invasive Prenatal Testing Market?
Key companies in the market include Natera Inc, Invitae Corporation, F Hoffmann-La Roche Ltd, Eurofins Scientific, Centogene NV, Myriad Womens Health Inc, BGI, Qiagen*List Not Exhaustive, PerkinElmer Inc, Illumina Inc, MedGenome Labs Ltd.
3. What are the main segments of the Non-invasive Prenatal Testing Market?
The market segments include Component, Application, End User.
4. Can you provide details about the market size?
The market size is estimated to be USD 6.28 billion as of 2022.
5. What are some drivers contributing to market growth?
Increasing Number of Babies with Chromosomal Disorders Owing to Increasing Number of Late Pregnancies; Increasing Demand for Early and Non-invasive Fetal Diagnosis; Favorable Reimbursement Policies.
6. What are the notable trends driving market growth?
Down Syndrome Segment Dominates the Non-invasive Prenatal Testing Market.
7. Are there any restraints impacting market growth?
Lack of Skilled Professionals; Stringent Regulations and Ethical Concerns.
8. Can you provide examples of recent developments in the market?
August 2022: Natera Inc. filed a pre-submission to the Food and Drug Administration (FDA) for its panorama non-invasive prenatal test (NIPT) as part of the Q-Sub process. The company filed its pre-submission in June 2022 for fetal chromosomal aneuploidies and 22q11.2 deletion syndrome.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in billion.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Non-invasive Prenatal Testing Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Non-invasive Prenatal Testing Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Non-invasive Prenatal Testing Market?
To stay informed about further developments, trends, and reports in the Non-invasive Prenatal Testing Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
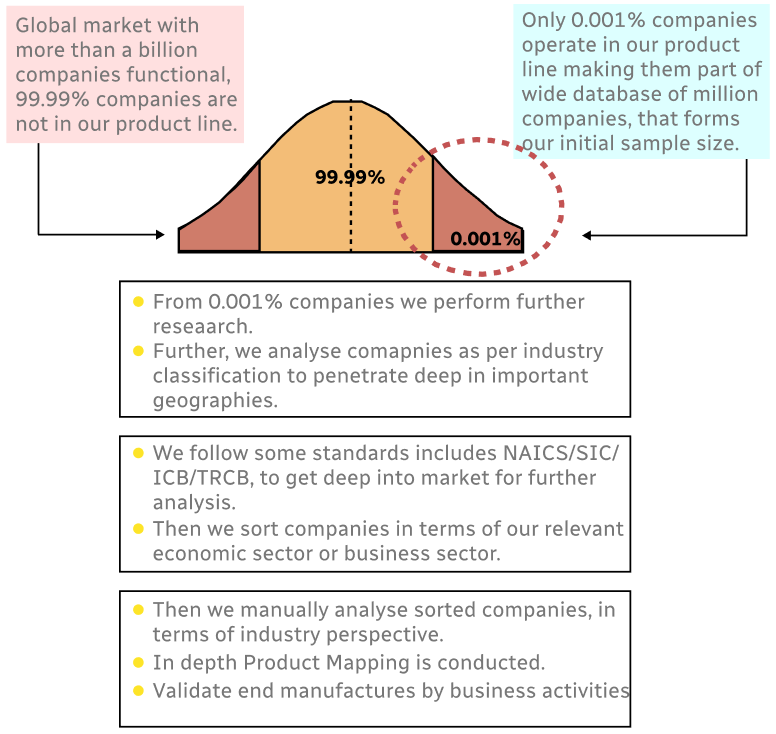
Step 1 - Identification of Relevant Samples Size from Population Database
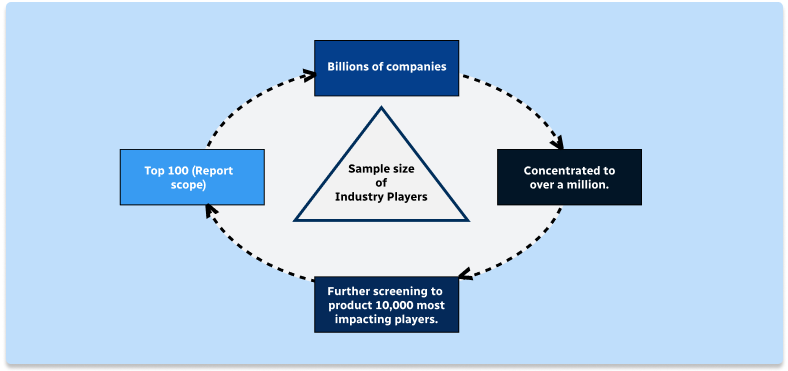
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
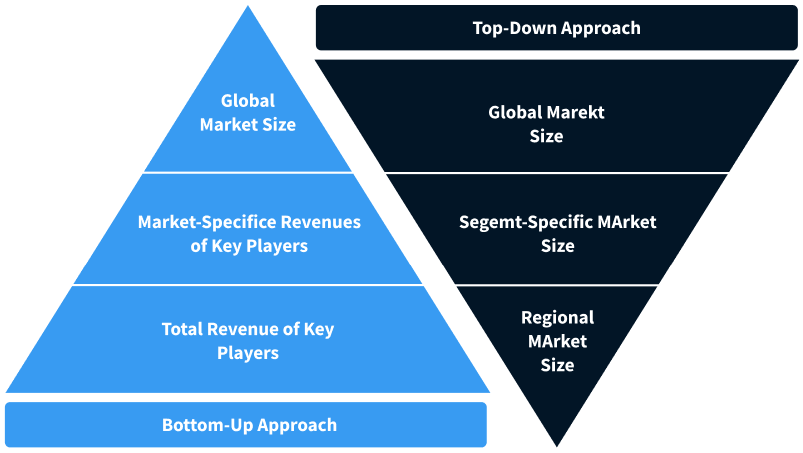
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
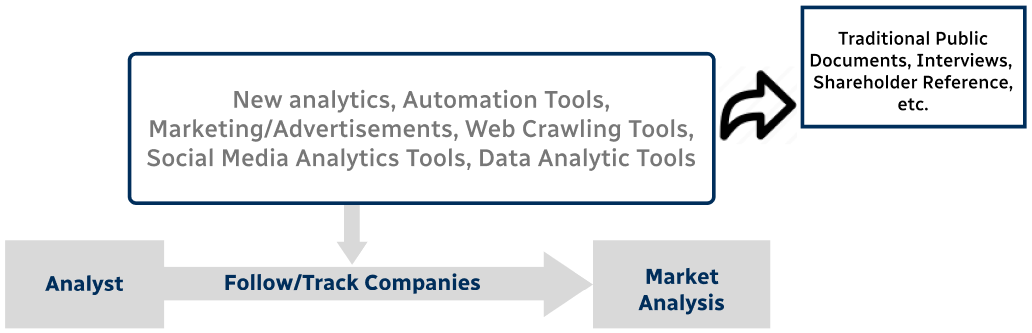
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


