Key Insights
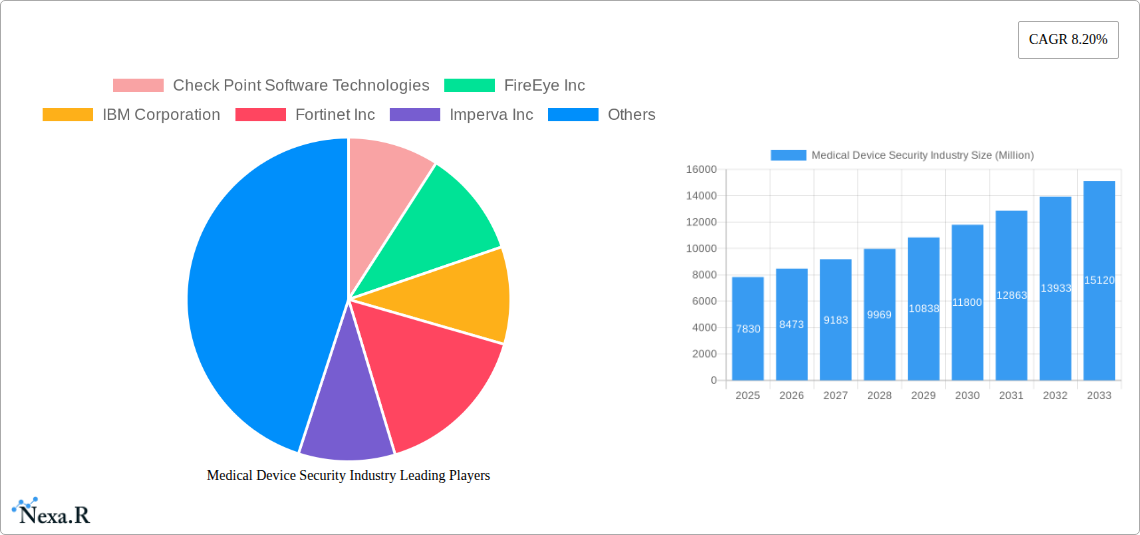
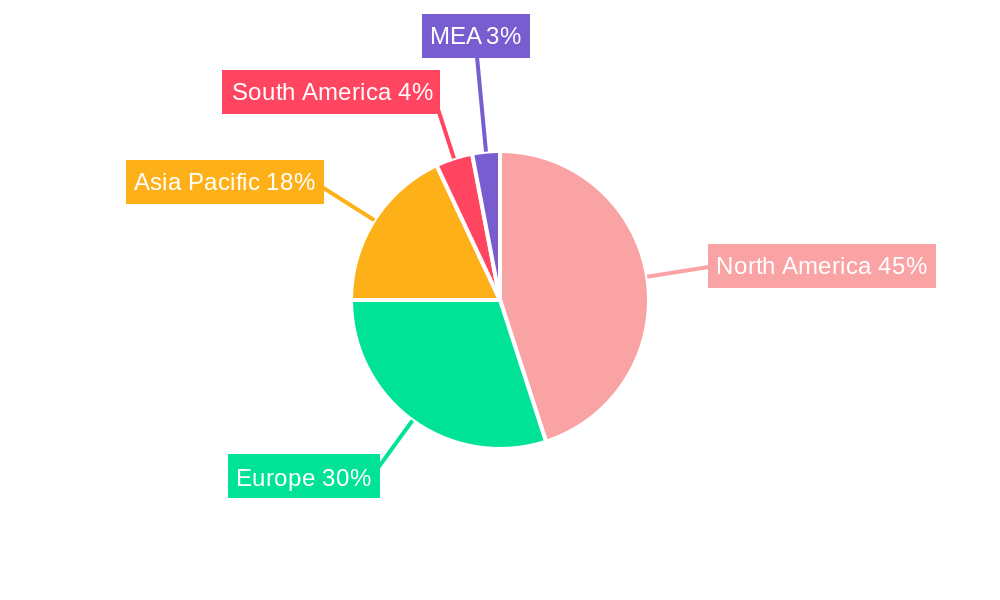
The medical device security market, valued at $7.83 billion in 2025, is experiencing robust growth, projected to expand at a compound annual growth rate (CAGR) of 8.20% from 2025 to 2033. This significant expansion is driven by several key factors. The increasing connectivity of medical devices through the Internet of Medical Things (IoMT) creates new vulnerabilities, necessitating robust security measures. Simultaneously, stringent regulatory compliance requirements, such as HIPAA and GDPR, are driving adoption of advanced security solutions. Rising cyberattacks targeting healthcare institutions, aiming to steal sensitive patient data or disrupt critical medical operations, further fuel market growth. The market is segmented by solution type (Data Loss Prevention, Antivirus/Antimalware, Encryption, Network & Endpoint Security, Identity & Access Management, Intrusion Detection/Prevention Systems, Risk & Compliance Management, and Other Solutions) and device type (Hospital Medical Devices, Internally Embedded Medical Devices, and Wearable/External Medical Devices). North America currently holds a dominant market share due to advanced technological infrastructure and high healthcare spending, followed by Europe and Asia Pacific. However, the Asia Pacific region is expected to witness significant growth in the coming years, driven by rising healthcare expenditure and increasing adoption of connected medical devices.
The competitive landscape is characterized by a mix of established cybersecurity companies and specialized medical device manufacturers. Key players such as Check Point, FireEye, IBM, Fortinet, Imperva, McAfee, Cisco, General Electric, Palo Alto Networks, and ClearDATA are actively investing in research and development to enhance their offerings and expand their market presence. Future growth will be influenced by the development and adoption of artificial intelligence (AI) and machine learning (ML) in security solutions, improved threat detection capabilities, and a greater focus on proactive security measures. The market also faces restraints, such as the high cost of implementation and maintenance of security solutions, as well as the complexity of integrating security across diverse medical device ecosystems. Despite these challenges, the continued growth of IoMT and increasing regulatory pressure will continue to propel the market's expansion in the forecast period.
Medical Device Security Industry Market Report: 2019-2033
This comprehensive report provides a detailed analysis of the Medical Device Security market, encompassing market size, growth trends, competitive landscape, and future outlook. With a study period spanning 2019-2033, a base year of 2025, and a forecast period of 2025-2033, this report is an invaluable resource for industry professionals, investors, and strategic decision-makers. The report segments the market by solution (Data Loss Prevention, Antivirus/Antimalware, Encryption, Network & Endpoint Security, Identity & Access Management, Intrusion Detection/Prevention, Risk & Compliance Management, Other) and device type (Hospital Medical Devices, Internally Embedded Medical Devices, Wearable & External Medical Devices). The market is valued in million units.
Medical Device Security Industry Market Dynamics & Structure
The medical device security market is characterized by increasing market concentration among major players, driven by technological innovation and stringent regulatory frameworks. The market is witnessing significant mergers and acquisitions (M&A) activity, with a projected xx% increase in deal volume between 2024 and 2028. The rise of connected medical devices and the increasing reliance on electronic health records (EHRs) are major drivers of growth, while high implementation costs and a lack of standardized security protocols pose significant challenges.
- Market Concentration: The top 5 players hold an estimated xx% market share in 2025.
- Technological Innovation: Advancements in AI-powered security solutions and blockchain technology are driving market growth.
- Regulatory Landscape: Compliance with HIPAA, GDPR, and other regulations is increasing the demand for robust security solutions.
- Competitive Substitutes: Limited substitutes exist due to the specialized nature of medical device security.
- End-User Demographics: Hospitals, clinics, and medical device manufacturers are the primary end-users.
- M&A Trends: A projected xx M&A deals are anticipated in the forecast period, driven by consolidation and expansion strategies.
Medical Device Security Industry Growth Trends & Insights
The global medical device security market is experiencing robust growth, with a projected Compound Annual Growth Rate (CAGR) of xx% during the forecast period (2025-2033). This growth is fueled by the increasing adoption of connected medical devices, rising cyber threats targeting healthcare systems, and the growing awareness of data privacy regulations. Market penetration of advanced security solutions remains relatively low, presenting significant growth opportunities. Technological disruptions, such as the rise of IoT security and AI-driven threat detection, are reshaping the market landscape. Consumer behavior is shifting towards a greater demand for transparent and secure medical device solutions. The market size is expected to reach xx million units by 2033, up from xx million units in 2025.
Dominant Regions, Countries, or Segments in Medical Device Security Industry
North America currently dominates the medical device security market, holding approximately xx% market share in 2025, driven by the high adoption of advanced technologies and stringent regulatory frameworks. Europe is expected to witness significant growth due to increasing government initiatives and rising cybersecurity awareness. Within the solution segment, Network and Endpoint Security holds the largest market share (xx%), followed by Data Loss Prevention Solutions (xx%). In terms of device type, Hospital Medical Devices dominate the market due to their critical role in healthcare delivery.
- Key Drivers: Stringent regulatory compliance, high adoption of connected medical devices, increased cybersecurity awareness.
- North America: Strong regulatory environment and high technology adoption driving market leadership.
- Europe: Growing government initiatives and increased cybersecurity awareness fuel market expansion.
- Asia-Pacific: High growth potential due to increasing healthcare expenditure and rising digitalization.
- Network & Endpoint Security: Largest market segment driven by the need to protect connected devices.
Medical Device Security Industry Product Landscape
The medical device security market offers a diverse range of solutions, including advanced encryption technologies, AI-powered threat detection systems, and robust access control mechanisms. These solutions are tailored to meet the specific security needs of various medical devices, ranging from wearable sensors to implantable devices. Innovation focuses on improving interoperability, reducing false positives, and enhancing usability while addressing the unique challenges associated with medical data security. The market sees a continuous trend towards cloud-based security solutions and integrated security platforms.
Key Drivers, Barriers & Challenges in Medical Device Security Industry
Key Drivers: The increasing prevalence of cyberattacks targeting healthcare organizations, stringent regulatory requirements, and the growing adoption of connected medical devices are key drivers of market growth. Government initiatives promoting cybersecurity in the healthcare sector further accelerate market expansion.
Challenges: High implementation costs, the complexity of integrating security solutions into existing medical device infrastructure, and the lack of skilled cybersecurity professionals pose significant barriers. Supply chain vulnerabilities and the evolving nature of cyber threats represent ongoing challenges. Estimated losses due to cyberattacks on medical devices are projected at xx million units annually.
Emerging Opportunities in Medical Device Security Industry
Emerging opportunities lie in the development of AI-powered security solutions, blockchain-based data security technologies, and the integration of security measures into the design phase of medical devices. Untapped markets in developing economies present significant potential for growth. Increasing demand for secure remote patient monitoring solutions and the development of security standards for wearable medical devices offer further opportunities.
Growth Accelerators in the Medical Device Security Industry
Strategic partnerships between medical device manufacturers, cybersecurity firms, and healthcare providers are accelerating market growth. Technological advancements, such as the development of more sophisticated threat detection systems and the integration of advanced encryption technologies, are driving innovation. Government initiatives to improve cybersecurity infrastructure in the healthcare sector are stimulating market expansion.
Key Players Shaping the Medical Device Security Industry Market
- Check Point Software Technologies
- FireEye Inc
- IBM Corporation
- Fortinet Inc
- Imperva Inc
- McAfee LLC
- Cisco Systems Inc
- General Electric Company
- Palo Alto Networks Inc
- ClearDATA
Notable Milestones in Medical Device Security Industry Sector
- November 2023: Ostium Group's partnership with Eastman for sustainable packaging in orthopedic implants highlights a focus on supply chain security.
- November 2022: Cybeats Technologies Corp.'s collaboration with Health-ISAC signifies a move towards collaborative cybersecurity solutions for medical device manufacturers and healthcare providers.
In-Depth Medical Device Security Industry Market Outlook
The medical device security market is poised for continued strong growth, driven by increasing connectivity, stringent regulations, and the rising sophistication of cyber threats. Strategic partnerships, technological advancements, and expansion into emerging markets will shape the future landscape. The market presents significant opportunities for companies that can develop innovative and effective solutions to address the unique security challenges of the healthcare industry.
Medical Device Security Industry Segmentation
-
1. Solution
- 1.1. Data Loss Prevention Solutions
- 1.2. Antivirus/Antimalware Solutions
- 1.3. Encryption Solutions
- 1.4. Network and Endpoint Security
- 1.5. Identity and Access Management Solutions
- 1.6. Intrusio
- 1.7. Risk and Compliance Management
- 1.8. Other Solutions
-
2. Device Type
- 2.1. Hospital Medical Devices
- 2.2. Internally Embedded Medical Devices
- 2.3. Wearable and External Medical Devices
Medical Device Security Industry Segmentation By Geography
- 1. North America
- 2. Europe
- 3. Asia Pacific
- 4. Latin America
- 5. Middle East
Medical Device Security Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 8.20% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Demand for Connected Medical Devices; Government Regulations and Need for Compliance
- 3.3. Market Restrains
- 3.3.1. High Initial Cost of 3D Motion Capture Software
- 3.4. Market Trends
- 3.4.1. Increasing Demand for Connected Medical Devices is Expected to Drive the Market Growth
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Medical Device Security Industry Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Solution
- 5.1.1. Data Loss Prevention Solutions
- 5.1.2. Antivirus/Antimalware Solutions
- 5.1.3. Encryption Solutions
- 5.1.4. Network and Endpoint Security
- 5.1.5. Identity and Access Management Solutions
- 5.1.6. Intrusio
- 5.1.7. Risk and Compliance Management
- 5.1.8. Other Solutions
- 5.2. Market Analysis, Insights and Forecast - by Device Type
- 5.2.1. Hospital Medical Devices
- 5.2.2. Internally Embedded Medical Devices
- 5.2.3. Wearable and External Medical Devices
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. Europe
- 5.3.3. Asia Pacific
- 5.3.4. Latin America
- 5.3.5. Middle East
- 5.1. Market Analysis, Insights and Forecast - by Solution
- 6. North America Medical Device Security Industry Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Solution
- 6.1.1. Data Loss Prevention Solutions
- 6.1.2. Antivirus/Antimalware Solutions
- 6.1.3. Encryption Solutions
- 6.1.4. Network and Endpoint Security
- 6.1.5. Identity and Access Management Solutions
- 6.1.6. Intrusio
- 6.1.7. Risk and Compliance Management
- 6.1.8. Other Solutions
- 6.2. Market Analysis, Insights and Forecast - by Device Type
- 6.2.1. Hospital Medical Devices
- 6.2.2. Internally Embedded Medical Devices
- 6.2.3. Wearable and External Medical Devices
- 6.1. Market Analysis, Insights and Forecast - by Solution
- 7. Europe Medical Device Security Industry Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Solution
- 7.1.1. Data Loss Prevention Solutions
- 7.1.2. Antivirus/Antimalware Solutions
- 7.1.3. Encryption Solutions
- 7.1.4. Network and Endpoint Security
- 7.1.5. Identity and Access Management Solutions
- 7.1.6. Intrusio
- 7.1.7. Risk and Compliance Management
- 7.1.8. Other Solutions
- 7.2. Market Analysis, Insights and Forecast - by Device Type
- 7.2.1. Hospital Medical Devices
- 7.2.2. Internally Embedded Medical Devices
- 7.2.3. Wearable and External Medical Devices
- 7.1. Market Analysis, Insights and Forecast - by Solution
- 8. Asia Pacific Medical Device Security Industry Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Solution
- 8.1.1. Data Loss Prevention Solutions
- 8.1.2. Antivirus/Antimalware Solutions
- 8.1.3. Encryption Solutions
- 8.1.4. Network and Endpoint Security
- 8.1.5. Identity and Access Management Solutions
- 8.1.6. Intrusio
- 8.1.7. Risk and Compliance Management
- 8.1.8. Other Solutions
- 8.2. Market Analysis, Insights and Forecast - by Device Type
- 8.2.1. Hospital Medical Devices
- 8.2.2. Internally Embedded Medical Devices
- 8.2.3. Wearable and External Medical Devices
- 8.1. Market Analysis, Insights and Forecast - by Solution
- 9. Latin America Medical Device Security Industry Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Solution
- 9.1.1. Data Loss Prevention Solutions
- 9.1.2. Antivirus/Antimalware Solutions
- 9.1.3. Encryption Solutions
- 9.1.4. Network and Endpoint Security
- 9.1.5. Identity and Access Management Solutions
- 9.1.6. Intrusio
- 9.1.7. Risk and Compliance Management
- 9.1.8. Other Solutions
- 9.2. Market Analysis, Insights and Forecast - by Device Type
- 9.2.1. Hospital Medical Devices
- 9.2.2. Internally Embedded Medical Devices
- 9.2.3. Wearable and External Medical Devices
- 9.1. Market Analysis, Insights and Forecast - by Solution
- 10. Middle East Medical Device Security Industry Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Solution
- 10.1.1. Data Loss Prevention Solutions
- 10.1.2. Antivirus/Antimalware Solutions
- 10.1.3. Encryption Solutions
- 10.1.4. Network and Endpoint Security
- 10.1.5. Identity and Access Management Solutions
- 10.1.6. Intrusio
- 10.1.7. Risk and Compliance Management
- 10.1.8. Other Solutions
- 10.2. Market Analysis, Insights and Forecast - by Device Type
- 10.2.1. Hospital Medical Devices
- 10.2.2. Internally Embedded Medical Devices
- 10.2.3. Wearable and External Medical Devices
- 10.1. Market Analysis, Insights and Forecast - by Solution
- 11. North America Medical Device Security Industry Analysis, Insights and Forecast, 2019-2031
- 11.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 11.1.1 United States
- 11.1.2 Canada
- 11.1.3 Mexico
- 12. Europe Medical Device Security Industry Analysis, Insights and Forecast, 2019-2031
- 12.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 12.1.1 Germany
- 12.1.2 United Kingdom
- 12.1.3 France
- 12.1.4 Spain
- 12.1.5 Italy
- 12.1.6 Spain
- 12.1.7 Belgium
- 12.1.8 Netherland
- 12.1.9 Nordics
- 12.1.10 Rest of Europe
- 13. Asia Pacific Medical Device Security Industry Analysis, Insights and Forecast, 2019-2031
- 13.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 13.1.1 China
- 13.1.2 Japan
- 13.1.3 India
- 13.1.4 South Korea
- 13.1.5 Southeast Asia
- 13.1.6 Australia
- 13.1.7 Indonesia
- 13.1.8 Phillipes
- 13.1.9 Singapore
- 13.1.10 Thailandc
- 13.1.11 Rest of Asia Pacific
- 14. South America Medical Device Security Industry Analysis, Insights and Forecast, 2019-2031
- 14.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 14.1.1 Brazil
- 14.1.2 Argentina
- 14.1.3 Peru
- 14.1.4 Chile
- 14.1.5 Colombia
- 14.1.6 Ecuador
- 14.1.7 Venezuela
- 14.1.8 Rest of South America
- 15. North America Medical Device Security Industry Analysis, Insights and Forecast, 2019-2031
- 15.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 15.1.1 United States
- 15.1.2 Canada
- 15.1.3 Mexico
- 16. MEA Medical Device Security Industry Analysis, Insights and Forecast, 2019-2031
- 16.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 16.1.1 United Arab Emirates
- 16.1.2 Saudi Arabia
- 16.1.3 South Africa
- 16.1.4 Rest of Middle East and Africa
- 17. Competitive Analysis
- 17.1. Global Market Share Analysis 2024
- 17.2. Company Profiles
- 17.2.1 Check Point Software Technologies
- 17.2.1.1. Overview
- 17.2.1.2. Products
- 17.2.1.3. SWOT Analysis
- 17.2.1.4. Recent Developments
- 17.2.1.5. Financials (Based on Availability)
- 17.2.2 FireEye Inc
- 17.2.2.1. Overview
- 17.2.2.2. Products
- 17.2.2.3. SWOT Analysis
- 17.2.2.4. Recent Developments
- 17.2.2.5. Financials (Based on Availability)
- 17.2.3 IBM Corporation
- 17.2.3.1. Overview
- 17.2.3.2. Products
- 17.2.3.3. SWOT Analysis
- 17.2.3.4. Recent Developments
- 17.2.3.5. Financials (Based on Availability)
- 17.2.4 Fortinet Inc
- 17.2.4.1. Overview
- 17.2.4.2. Products
- 17.2.4.3. SWOT Analysis
- 17.2.4.4. Recent Developments
- 17.2.4.5. Financials (Based on Availability)
- 17.2.5 Imperva Inc
- 17.2.5.1. Overview
- 17.2.5.2. Products
- 17.2.5.3. SWOT Analysis
- 17.2.5.4. Recent Developments
- 17.2.5.5. Financials (Based on Availability)
- 17.2.6 McAfee LLC
- 17.2.6.1. Overview
- 17.2.6.2. Products
- 17.2.6.3. SWOT Analysis
- 17.2.6.4. Recent Developments
- 17.2.6.5. Financials (Based on Availability)
- 17.2.7 Cisco Systems Inc
- 17.2.7.1. Overview
- 17.2.7.2. Products
- 17.2.7.3. SWOT Analysis
- 17.2.7.4. Recent Developments
- 17.2.7.5. Financials (Based on Availability)
- 17.2.8 General Electric Company
- 17.2.8.1. Overview
- 17.2.8.2. Products
- 17.2.8.3. SWOT Analysis
- 17.2.8.4. Recent Developments
- 17.2.8.5. Financials (Based on Availability)
- 17.2.9 Palo Alto Networks Inc
- 17.2.9.1. Overview
- 17.2.9.2. Products
- 17.2.9.3. SWOT Analysis
- 17.2.9.4. Recent Developments
- 17.2.9.5. Financials (Based on Availability)
- 17.2.10 ClearDATA
- 17.2.10.1. Overview
- 17.2.10.2. Products
- 17.2.10.3. SWOT Analysis
- 17.2.10.4. Recent Developments
- 17.2.10.5. Financials (Based on Availability)
- 17.2.1 Check Point Software Technologies
List of Figures
- Figure 1: Global Medical Device Security Industry Revenue Breakdown (Million, %) by Region 2024 & 2032
- Figure 2: North America Medical Device Security Industry Revenue (Million), by Country 2024 & 2032
- Figure 3: North America Medical Device Security Industry Revenue Share (%), by Country 2024 & 2032
- Figure 4: Europe Medical Device Security Industry Revenue (Million), by Country 2024 & 2032
- Figure 5: Europe Medical Device Security Industry Revenue Share (%), by Country 2024 & 2032
- Figure 6: Asia Pacific Medical Device Security Industry Revenue (Million), by Country 2024 & 2032
- Figure 7: Asia Pacific Medical Device Security Industry Revenue Share (%), by Country 2024 & 2032
- Figure 8: South America Medical Device Security Industry Revenue (Million), by Country 2024 & 2032
- Figure 9: South America Medical Device Security Industry Revenue Share (%), by Country 2024 & 2032
- Figure 10: North America Medical Device Security Industry Revenue (Million), by Country 2024 & 2032
- Figure 11: North America Medical Device Security Industry Revenue Share (%), by Country 2024 & 2032
- Figure 12: MEA Medical Device Security Industry Revenue (Million), by Country 2024 & 2032
- Figure 13: MEA Medical Device Security Industry Revenue Share (%), by Country 2024 & 2032
- Figure 14: North America Medical Device Security Industry Revenue (Million), by Solution 2024 & 2032
- Figure 15: North America Medical Device Security Industry Revenue Share (%), by Solution 2024 & 2032
- Figure 16: North America Medical Device Security Industry Revenue (Million), by Device Type 2024 & 2032
- Figure 17: North America Medical Device Security Industry Revenue Share (%), by Device Type 2024 & 2032
- Figure 18: North America Medical Device Security Industry Revenue (Million), by Country 2024 & 2032
- Figure 19: North America Medical Device Security Industry Revenue Share (%), by Country 2024 & 2032
- Figure 20: Europe Medical Device Security Industry Revenue (Million), by Solution 2024 & 2032
- Figure 21: Europe Medical Device Security Industry Revenue Share (%), by Solution 2024 & 2032
- Figure 22: Europe Medical Device Security Industry Revenue (Million), by Device Type 2024 & 2032
- Figure 23: Europe Medical Device Security Industry Revenue Share (%), by Device Type 2024 & 2032
- Figure 24: Europe Medical Device Security Industry Revenue (Million), by Country 2024 & 2032
- Figure 25: Europe Medical Device Security Industry Revenue Share (%), by Country 2024 & 2032
- Figure 26: Asia Pacific Medical Device Security Industry Revenue (Million), by Solution 2024 & 2032
- Figure 27: Asia Pacific Medical Device Security Industry Revenue Share (%), by Solution 2024 & 2032
- Figure 28: Asia Pacific Medical Device Security Industry Revenue (Million), by Device Type 2024 & 2032
- Figure 29: Asia Pacific Medical Device Security Industry Revenue Share (%), by Device Type 2024 & 2032
- Figure 30: Asia Pacific Medical Device Security Industry Revenue (Million), by Country 2024 & 2032
- Figure 31: Asia Pacific Medical Device Security Industry Revenue Share (%), by Country 2024 & 2032
- Figure 32: Latin America Medical Device Security Industry Revenue (Million), by Solution 2024 & 2032
- Figure 33: Latin America Medical Device Security Industry Revenue Share (%), by Solution 2024 & 2032
- Figure 34: Latin America Medical Device Security Industry Revenue (Million), by Device Type 2024 & 2032
- Figure 35: Latin America Medical Device Security Industry Revenue Share (%), by Device Type 2024 & 2032
- Figure 36: Latin America Medical Device Security Industry Revenue (Million), by Country 2024 & 2032
- Figure 37: Latin America Medical Device Security Industry Revenue Share (%), by Country 2024 & 2032
- Figure 38: Middle East Medical Device Security Industry Revenue (Million), by Solution 2024 & 2032
- Figure 39: Middle East Medical Device Security Industry Revenue Share (%), by Solution 2024 & 2032
- Figure 40: Middle East Medical Device Security Industry Revenue (Million), by Device Type 2024 & 2032
- Figure 41: Middle East Medical Device Security Industry Revenue Share (%), by Device Type 2024 & 2032
- Figure 42: Middle East Medical Device Security Industry Revenue (Million), by Country 2024 & 2032
- Figure 43: Middle East Medical Device Security Industry Revenue Share (%), by Country 2024 & 2032
List of Tables
- Table 1: Global Medical Device Security Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Global Medical Device Security Industry Revenue Million Forecast, by Solution 2019 & 2032
- Table 3: Global Medical Device Security Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 4: Global Medical Device Security Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 5: Global Medical Device Security Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 6: United States Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 7: Canada Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 8: Mexico Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 9: Global Medical Device Security Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 10: Germany Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 11: United Kingdom Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 12: France Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 13: Spain Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 14: Italy Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 15: Spain Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 16: Belgium Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 17: Netherland Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 18: Nordics Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 19: Rest of Europe Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 20: Global Medical Device Security Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 21: China Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 22: Japan Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 23: India Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 24: South Korea Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 25: Southeast Asia Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 26: Australia Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 27: Indonesia Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 28: Phillipes Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 29: Singapore Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 30: Thailandc Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 31: Rest of Asia Pacific Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 32: Global Medical Device Security Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 33: Brazil Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 34: Argentina Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 35: Peru Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 36: Chile Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 37: Colombia Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 38: Ecuador Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 39: Venezuela Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 40: Rest of South America Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 41: Global Medical Device Security Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 42: United States Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 43: Canada Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 44: Mexico Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 45: Global Medical Device Security Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 46: United Arab Emirates Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 47: Saudi Arabia Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 48: South Africa Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 49: Rest of Middle East and Africa Medical Device Security Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 50: Global Medical Device Security Industry Revenue Million Forecast, by Solution 2019 & 2032
- Table 51: Global Medical Device Security Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 52: Global Medical Device Security Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 53: Global Medical Device Security Industry Revenue Million Forecast, by Solution 2019 & 2032
- Table 54: Global Medical Device Security Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 55: Global Medical Device Security Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 56: Global Medical Device Security Industry Revenue Million Forecast, by Solution 2019 & 2032
- Table 57: Global Medical Device Security Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 58: Global Medical Device Security Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 59: Global Medical Device Security Industry Revenue Million Forecast, by Solution 2019 & 2032
- Table 60: Global Medical Device Security Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 61: Global Medical Device Security Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 62: Global Medical Device Security Industry Revenue Million Forecast, by Solution 2019 & 2032
- Table 63: Global Medical Device Security Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 64: Global Medical Device Security Industry Revenue Million Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Medical Device Security Industry?
The projected CAGR is approximately 8.20%.
2. Which companies are prominent players in the Medical Device Security Industry?
Key companies in the market include Check Point Software Technologies, FireEye Inc, IBM Corporation, Fortinet Inc, Imperva Inc, McAfee LLC, Cisco Systems Inc, General Electric Company, Palo Alto Networks Inc, ClearDATA.
3. What are the main segments of the Medical Device Security Industry?
The market segments include Solution, Device Type.
4. Can you provide details about the market size?
The market size is estimated to be USD 7.83 Million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Demand for Connected Medical Devices; Government Regulations and Need for Compliance.
6. What are the notable trends driving market growth?
Increasing Demand for Connected Medical Devices is Expected to Drive the Market Growth.
7. Are there any restraints impacting market growth?
High Initial Cost of 3D Motion Capture Software.
8. Can you provide examples of recent developments in the market?
November 2023: Ostium Group, one of the global leaders of medical device startups dedicated to revolutionizing orthopedic surgical instrumentation, announced its strategic partnership with Eastman, a global specialty materials company. The collaboration focuses on incorporating Eastman's sustainable packaging solution, Eastar 6763 Renew copolyester, into Ostium Group's innovative CILLAR Acetabular and Femoral kits for total hip replacements.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Medical Device Security Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Medical Device Security Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Medical Device Security Industry?
To stay informed about further developments, trends, and reports in the Medical Device Security Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


